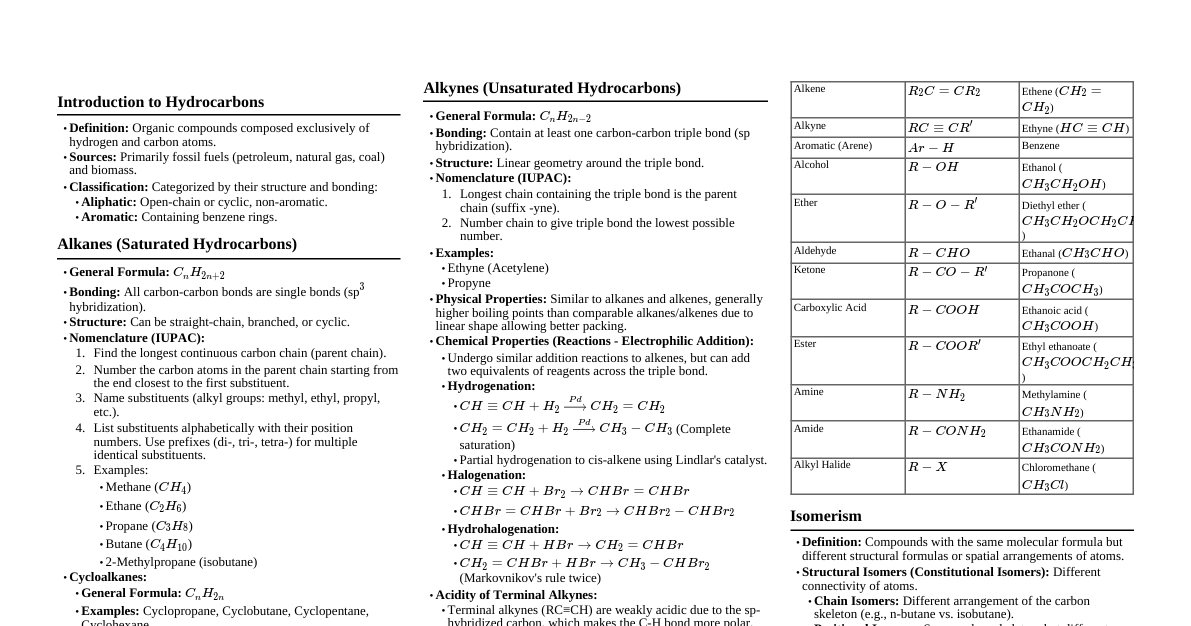
### Introduction Hydrocarbons are organic compounds consisting entirely of hydrogen and carbon atoms. They form the basis of organic chemistry and are crucial as fuels and raw materials for many industrial applications. This cheatsheet covers their classification, preparation, properties, and important reactions. **Key Terms:** - **LPG (Liquefied Petroleum Gas):** Fuel, mixture of hydrocarbons. - **CNG (Compressed Natural Gas):** Fuel, compressed natural gas. - **LNG (Liquefied Natural Gas):** Fuel, liquefied natural gas. - **Petroleum:** Source of petrol, diesel, kerosene through fractional distillation. - **Polymers:** Hydrocarbons are used to manufacture plastics like polythene, polypropene, polystyrene. ### Classification of Hydrocarbons Hydrocarbons are classified based on the types of carbon-carbon bonds present: 1. **Saturated Hydrocarbons:** Contain only carbon-carbon single bonds. * **Alkanes:** Open-chain hydrocarbons (e.g., methane, ethane). * **Cycloalkanes:** Closed-chain or ring hydrocarbons (e.g., cyclopropane, cyclohexane). 2. **Unsaturated Hydrocarbons:** Contain carbon-carbon multiple bonds (double or triple). * **Alkenes:** Contain at least one C=C double bond. * **Alkynes:** Contain at least one C≡C triple bond. 3. **Aromatic Hydrocarbons:** Special type of cyclic compounds, typically containing a benzene ring, exhibiting aromaticity. **Carbon Valency:** Carbon is tetravalent, hydrogen is monovalent. **General Formulas:** - **Alkanes:** $\text{C}_n\text{H}_{2n+2}$ - **Alkenes:** $\text{C}_n\text{H}_{2n}$ (for one double bond) - **Alkynes:** $\text{C}_n\text{H}_{2n-2}$ (for one triple bond) ### Alkanes: Nomenclature & Isomerism **Nomenclature (IUPAC):** - **Parent Chain:** Longest continuous carbon chain. - **Substituents:** Alkyl groups attached to the parent chain. - **Numbering:** Number the chain to give substituents the lowest possible numbers. - **Alphabetical Order:** List substituents alphabetically (ignoring prefixes like di-, tri-, sec-, tert-). - **Prefixes:** Use di-, tri-, tetra- for multiple identical substituents. **Isomerism:** - **Structural Isomers:** Compounds with the same molecular formula but different structural arrangements. * **Chain Isomerism:** Different arrangement of carbon atoms in the chain (e.g., n-butane vs. isobutane). * **Position Isomerism:** Different position of a substituent or functional group on the same carbon skeleton. **Alkyl Groups:** Derived from alkanes by removing one hydrogen atom ($\text{C}_n\text{H}_{2n+1}$). - Examples: Methyl ($\text{-CH}_3$), Ethyl ($\text{-CH}_2\text{CH}_3$), Propyl ($\text{-CH}_2\text{CH}_2\text{CH}_3$), Isopropyl ($\text{-CH(CH}_3\text{)}_2$). **Types of Carbon Atoms:** - **Primary (1°):** Attached to no other C or one other C (terminal). - **Secondary (2°):** Attached to two other C atoms. - **Tertiary (3°):** Attached to three other C atoms. - **Quaternary (4°):** Attached to four other C atoms. **Example (C6H14 isomers):** 1. **n-Hexane:** $\text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{CH}_2\text{CH}_3$ 2. **2-Methylpentane:** $\text{CH}_3\text{CH(CH}_3)\text{CH}_2\text{CH}_2\text{CH}_3$ 3. **3-Methylpentane:** $\text{CH}_3\text{CH}_2\text{CH(CH}_3)\text{CH}_2\text{CH}_3$ 4. **2,3-Dimethylbutane:** $\text{CH}_3\text{CH(CH}_3)\text{CH(CH}_3)\text{CH}_3$ 5. **2,2-Dimethylbutane:** $\text{CH}_3\text{C(CH}_3\text{)}_2\text{CH}_2\text{CH}_3$ ### Alkanes: Preparation 1. **From Unsaturated Hydrocarbons (Hydrogenation):** * Alkenes/Alkynes + H₂ $\xrightarrow{\text{Pt/Pd/Ni}}$ Alkanes * Example: $\text{CH}_2=\text{CH}_2 + \text{H}_2 \xrightarrow{\text{Ni}}$ $\text{CH}_3\text{-CH}_3$ (Ethene to Ethane) * Catalysts (Pt, Pd, Ni) adsorb H₂ and activate H-H bond. 2. **From Alkyl Halides:** * **Reduction:** Alkyl Halide + H₂ $\xrightarrow{\text{Zn, H}^+}$ Alkane + HX * Example: $\text{CH}_3\text{Cl} + \text{H}_2 \xrightarrow{\text{Zn, H}^+}$ $\text{CH}_4 + \text{HCl}$ (Chloromethane to Methane) * **Wurtz Reaction:** Alkyl Halide + Na $\xrightarrow{\text{dry ether}}$ Higher Alkane (even number of C atoms) * Example: $2\text{CH}_3\text{Br} + 2\text{Na} \xrightarrow{\text{dry ether}}$ $\text{CH}_3\text{-CH}_3 + 2\text{NaBr}$ (Bromomethane to Ethane) * Not suitable for odd-numbered alkanes due to mixture formation. 3. **From Carboxylic Acids:** * **Decarboxylation:** Sodium salt of carboxylic acid + Soda lime (NaOH + CaO) $\xrightarrow{\Delta}$ Alkane (one C less) + $\text{Na}_2\text{CO}_3$ * Example: $\text{CH}_3\text{COO}^-\text{Na}^+ + \text{NaOH} \xrightarrow{\text{CaO, }\Delta}$ $\text{CH}_4 + \text{Na}_2\text{CO}_3$ (Sodium ethanoate to Methane) * **Kolbe's Electrolytic Method:** Aqueous solution of sodium/potassium salt of carboxylic acid $\xrightarrow{\text{electrolysis}}$ Alkane (even number of C atoms at anode) * Example: $2\text{CH}_3\text{COO}^-\text{Na}^+ \xrightarrow{\text{electrolysis}}$ $\text{CH}_3\text{-CH}_3 + 2\text{CO}_2 + \text{H}_2 + 2\text{NaOH}$ (Sodium acetate to Ethane) * Methane cannot be prepared by this method. ### Alkanes: Physical & Chemical Properties **Physical Properties:** - **Non-polar:** Due to covalent C-C and C-H bonds and small electronegativity difference. - **Van der Waals forces:** Weak intermolecular forces. - **State:** $\text{C}_1$-$\text{C}_4$ are gases, $\text{C}_5$-$\text{C}_{17}$ are liquids, $\text{C}_{18+}$ are solids. - **Solubility:** Insoluble in water (hydrophobic), soluble in non-polar solvents ("like dissolves like"). - **Boiling Point (b.p.):** Increases with molecular mass (stronger van der Waals forces). - **Branching Effect:** Branched alkanes have lower b.p. than straight-chain isomers (smaller surface area, weaker forces). **Chemical Properties:** - Generally inert towards acids, bases, oxidizing/reducing agents ("paraffins"). - Undergo reactions under specific conditions: 1. **Substitution Reactions (Halogenation):** Replacement of H atoms by halogens, nitro, or sulfonic acid groups. * $\text{CH}_4 + \text{Cl}_2 \xrightarrow{\text{hv or }\Delta}$ $\text{CH}_3\text{Cl} + \text{HCl}$ (Chlorination of methane) * Occurs via free radical chain mechanism (Initiation, Propagation, Termination). * Reactivity of halogens: $\text{F}_2 > \text{Cl}_2 > \text{Br}_2 > \text{I}_2$. * Reactivity of H atoms: 3° > 2° > 1°. 2. **Combustion:** Alkanes burn in air/O₂ to produce $\text{CO}_2$ and $\text{H}_2\text{O}$ with heat (fuels). * Complete: $\text{CH}_4(\text{g}) + 2\text{O}_2(\text{g}) \rightarrow \text{CO}_2(\text{g}) + 2\text{H}_2\text{O}(\text{l})$ ($\Delta\text{H} = -890 \text{ kJ mol}^{-1}$) * Incomplete: Forms carbon black/soot (used in ink, pigments). 3. **Controlled Oxidation:** With specific catalysts and conditions. * $\text{2CH}_4 + \text{O}_2 \xrightarrow{\text{Cu/523K/100 atm}}$ $\text{2CH}_3\text{OH}$ (Methanol) * $\text{CH}_4 + \text{O}_2 \xrightarrow{\text{Mo}_2\text{O}_3/\Delta}$ $\text{HCHO} + \text{H}_2\text{O}$ (Methanal) * Alkanes with tertiary H can be oxidized to alcohols by $\text{KMnO}_4$. 4. **Isomerization:** n-Alkanes $\xrightarrow{\text{Anhy. AlCl}_3/\text{HCl}}$ Branched-chain alkanes. 5. **Aromatization (Reforming):** n-Alkanes ($\text{C}_{6+}$) $\xrightarrow{\text{Cr}_2\text{O}_3/\text{V}_2\text{O}_5/\text{Mo}_2\text{O}_3, 773\text{K}}$ Benzene and its homologues. 6. **Reaction with Steam:** $\text{CH}_4 + \text{H}_2\text{O} \xrightarrow{\text{Ni}, 1273\text{K}}$ $\text{CO} + 3\text{H}_2$ (Industrial $\text{H}_2$ prep.) 7. **Pyrolysis (Cracking):** Higher alkanes $\xrightarrow{\text{heat}}$ Lower alkanes, alkenes (free radical mechanism). * Used to produce oil gas, petrol gas from kerosene oil. ### Conformations of Ethane - **Conformations (Conformers/Rotamers):** Different spatial arrangements that can interconvert by rotation around a C-C single bond. - Rotation around C-C single bonds is not completely free due to **torsional strain** (weak repulsive interactions between adjacent bonds). - **Ethane ($\text{C}_2\text{H}_6$):** Contains one C-C single bond. * **Eclipsed Conformation:** Hydrogen atoms on adjacent carbons are as close as possible. High torsional strain, higher energy, less stable. * **Staggered Conformation:** Hydrogen atoms on adjacent carbons are as far apart as possible. Minimum torsional strain, lower energy, most stable. * **Skew Conformation:** Any intermediate conformation. **Representations:** - **Sawhorse Projections:** Views the molecule along the C-C bond, drawing it as a longer straight line. Front carbon at lower end, rear carbon at upper end. * *Eclipsed:* H atoms directly aligned. * *Staggered:* H atoms offset. - **Newman Projections:** Views the molecule head-on along the C-C bond. Front carbon as a point, rear carbon as a circle. * *Eclipsed:* H atoms of front carbon directly overlap H atoms of rear carbon. * *Staggered:* H atoms of front carbon bisect the angle between H atoms of rear carbon. **Relative Stability:** Staggered > Skew > Eclipsed. Energy difference is small (~12.5 kJ mol⁻¹), so rotation is considered essentially free at ordinary temperatures. ### Alkenes: Structure & Properties **Structure of Double Bond:** - Consists of one $\sigma$ bond and one $\pi$ bond. * **$\sigma$ bond:** Formed by head-on overlap of $\text{sp}^2$ hybrid orbitals of carbon. * **$\pi$ bond:** Formed by lateral/sideways overlap of unhybridized p orbitals. - **Bond Length:** C=C bond (134 pm) is shorter than C-C single bond (154 pm). - **Bond Strength:** C=C double bond (681 kJ mol⁻¹) is stronger than C-C single bond (348 kJ mol⁻¹), but the $\pi$ bond is weaker than the $\sigma$ bond. - **Reactivity:** $\pi$ electrons are loosely held, making alkenes electron-rich and susceptible to **electrophilic attack**. **Nomenclature (IUPAC):** - **Parent Chain:** Longest carbon chain containing the double bond. - **Suffix:** '-ene' replaces '-ane'. - **Numbering:** Start from the end nearer to the double bond to give it the lowest possible number. - First member: Ethene ($\text{C}_2\text{H}_4$). Methane ($\text{CH}_2$) is very unstable. **Isomerism:** - **Structural Isomerism:** * **Chain Isomerism:** Different carbon chain arrangements. * **Position Isomerism:** Different position of the double bond. - **Geometrical (cis-trans) Isomerism:** Arises due to restricted rotation around the C=C double bond. * Requires two different atoms/groups attached to each carbon of the double bond. * **Cis Isomer:** Identical atoms/groups are on the same side of the double bond. * **Trans Isomer:** Identical atoms/groups are on opposite sides of the double bond. * **Properties:** Cis and trans isomers have different physical properties (melting point, boiling point, dipole moment). Cis forms are generally more polar and have higher boiling points. Trans forms are often more stable. * Example: But-2-ene (cis-but-2-ene vs. trans-but-2-ene). ### Alkenes: Preparation 1. **From Alkynes (Partial Reduction):** * Alkyne + H₂ $\xrightarrow{\text{Lindlar's catalyst (Pd/CaCO}_3 + \text{quinoline/sulfur)}}$ Cis-Alkene * Alkyne + H₂ $\xrightarrow{\text{Na/liquid NH}_3}$ Trans-Alkene * Example: $\text{CH}_3\text{-C}\equiv\text{C-H} + \text{H}_2 \xrightarrow{\text{Lindlar's}}$ $\text{CH}_3\text{CH}=\text{CH}_2$ (Propyne to Propene) 2. **From Alkyl Halides (Dehydrohalogenation):** * Alkyl Halide + alcoholic KOH $\xrightarrow{\Delta}$ Alkene + KX + $\text{H}_2\text{O}$ ($\beta$-elimination) * Hydrogen is removed from the $\beta$-carbon (adjacent to the carbon with halogen). * Reactivity of halogens: $\text{I} > \text{Br} > \text{Cl}$. * Reactivity of alkyl groups: 3° > 2° > 1°. 3. **From Vicinal Dihalides (Dehalogenation):** * Vicinal Dihalide (halogens on adjacent carbons) + Zn $\rightarrow$ Alkene + $\text{ZnX}_2$ * Example: $\text{CH}_2\text{Br-CH}_2\text{Br} + \text{Zn} \rightarrow \text{CH}_2=\text{CH}_2 + \text{ZnBr}_2$ 4. **From Alcohols (Acidic Dehydration):** * Alcohol $\xrightarrow{\text{conc. H}_2\text{SO}_4/\Delta}$ Alkene + $\text{H}_2\text{O}$ ($\beta$-elimination) * Example: $\text{CH}_3\text{CH}_2\text{OH} \xrightarrow{\text{conc. H}_2\text{SO}_4/\Delta}$ $\text{CH}_2=\text{CH}_2 + \text{H}_2\text{O}$ (Ethanol to Ethene) ### Alkenes: Reactions **Chemical Properties:** - Rich source of $\pi$ electrons, undergo primarily **addition reactions** (electrophilic). - Some free radical substitution, oxidation, ozonolysis also occur. 1. **Addition of Dihydrogen (Hydrogenation):** * Alkene + H₂ $\xrightarrow{\text{Ni/Pt/Pd}}$ Alkane (Same as preparation, but in reverse) 2. **Addition of Halogens:** * Alkene + $\text{X}_2$ (e.g., $\text{Br}_2/\text{CCl}_4$) $\rightarrow$ Vicinal Dihalide (electrophilic addition) * Example: $\text{CH}_2=\text{CH}_2 + \text{Br}_2 \rightarrow \text{CH}_2\text{Br-CH}_2\text{Br}$ (Test for unsaturation; reddish-brown $\text{Br}_2$ color disappears). 3. **Addition of Hydrogen Halides (HX):** * Alkene + HX $\rightarrow$ Alkyl Halide (electrophilic addition) * Reactivity of HX: $\text{HI} > \text{HBr} > \text{HCl}$. * **Symmetrical Alkenes:** Only one product. * **Unsymmetrical Alkenes (Markovnikov's Rule):** The negative part of the addendum (X) attaches to the carbon atom with fewer hydrogen atoms. * Example: $\text{CH}_3\text{CH}=\text{CH}_2 + \text{HBr} \rightarrow \text{CH}_3\text{CH(Br)CH}_3$ (2-Bromopropane, major product). * Mechanism involves carbocation formation (more stable carbocation forms faster). * **Anti-Markovnikov Addition (Peroxide Effect/Kharash Effect):** Only with HBr in presence of peroxides. * The negative part of the addendum (Br) attaches to the carbon atom with more hydrogen atoms. * Example: $\text{CH}_3\text{CH}=\text{CH}_2 + \text{HBr} \xrightarrow{\text{peroxide}}$ $\text{CH}_3\text{CH}_2\text{CH}_2\text{Br}$ (1-Bromopropane, major product). * Mechanism involves free radical chain reaction. 4. **Addition of Sulphuric Acid:** * Alkene + cold, conc. $\text{H}_2\text{SO}_4 \rightarrow$ Alkyl Hydrogen Sulphate (Markovnikov's rule applies). * Example: $\text{CH}_2=\text{CH}_2 + \text{H-OSO}_2\text{OH} \rightarrow \text{CH}_3\text{CH}_2\text{OSO}_2\text{OH}$ (Ethyl hydrogen sulphate). 5. **Addition of Water (Hydration):** * Alkene + $\text{H}_2\text{O} \xrightarrow{\text{H}_2\text{SO}_4}$ Alcohol (Markovnikov's rule applies). * Example: $\text{CH}_3\text{CH}=\text{CH}_2 + \text{H}_2\text{O} \xrightarrow{\text{H}^+}$ $\text{CH}_3\text{CH(OH)CH}_3$ (Propan-2-ol). 6. **Oxidation:** * **Baeyer's Reagent (cold, dilute, aqueous $\text{KMnO}_4$):** Alkene $\rightarrow$ Vicinal Diol (Glycol) * Example: $\text{CH}_2=\text{CH}_2 \xrightarrow{\text{Baeyer's Reagent}}$ $\text{CH}_2\text{OH-CH}_2\text{OH}$ (Ethane-1,2-diol). * **Acidic $\text{KMnO}_4$ or $\text{K}_2\text{Cr}_2\text{O}_7$:** Strong oxidation, cleaves double bond, forms ketones/acids. 7. **Ozonolysis:** Alkene + $\text{O}_3 \rightarrow$ Ozonide $\xrightarrow{\text{Zn/H}_2\text{O}}$ Aldehydes/Ketones. * Used to determine the position of the double bond. * Example: $\text{CH}_3\text{CH}=\text{CH}_2 + \text{O}_3 \rightarrow \text{Ozonide} \xrightarrow{\text{Zn/H}_2\text{O}}$ $\text{CH}_3\text{CHO} + \text{HCHO}$ (Ethanal + Methanal). 8. **Polymerization:** Alkenes (monomers) combine to form large molecules (polymers). * Example: n$\text{CH}_2=\text{CH}_2 \xrightarrow{\text{high T/P, catalyst}}$ $(-\text{CH}_2\text{-CH}_2-)_n$ (Polythene). ### Alkynes: Nomenclature & Isomerism **Nomenclature (IUPAC):** - **Parent Chain:** Longest carbon chain containing the triple bond. - **Suffix:** '-yne' replaces '-ane'. - **Numbering:** Start from the end nearer to the triple bond to give it the lowest possible number. - First member: Ethyne ($\text{C}_2\text{H}_2$, acetylene). **Isomerism:** - **Structural Isomerism:** * **Position Isomerism:** Different position of the triple bond (e.g., but-1-yne vs. but-2-yne). * **Chain Isomerism:** Different carbon chain arrangements. ### Alkynes: Structure & Properties **Structure of Triple Bond:** - Consists of one $\sigma$ bond and two $\pi$ bonds. * **$\sigma$ bond:** Formed by head-on overlap of sp hybrid orbitals of carbon. * **$\pi$ bonds:** Formed by lateral/sideways overlap of two sets of unhybridized p orbitals. - **Geometry:** Linear molecule (H-C≡C-H bond angle is 180°). - **Bond Length:** C≡C bond (120 pm) is shorter than C=C (134 pm) and C-C (154 pm). - **Bond Strength:** C≡C triple bond (823 kJ mol⁻¹) is stronger than double and single bonds. - **Electron Cloud:** Cylindrically symmetrical around the internuclear axis. **Physical Properties:** - Similar trends to alkanes and alkenes (gases, liquids, solids). - Colourless. Ethyne has a characteristic odor. - Weakly polar, lighter than water, immiscible with water, soluble in organic solvents. - Melting point, boiling point, and density increase with molar mass. **Chemical Properties:** - Undergo addition reactions due to $\pi$ bonds. - Exhibit acidic character due to sp hybridization. **Acidic Character of Alkyne:** - H atoms attached to sp hybridized carbon atoms are acidic. - **Reason:** sp hybrid orbitals have 50% s-character, making them more electronegative. This attracts the shared electron pair of the C-H bond more strongly, making the H atom more proton-like. - **Reactions:** * Alkynes + Na metal $\rightarrow$ Sodium acetylide + $\text{H}_2$ * Alkynes + Sodamide ($\text{NaNH}_2$) $\rightarrow$ Sodium acetylide + $\text{NH}_3$ - **Trend in Acidity:** Terminal Alkyne ($\text{HC}\equiv\text{CR}$) > Alkene > Alkane. * Internal alkynes ($\text{RC}\equiv\text{CR}$) do not show acidic character as they lack H atoms on the sp carbons. - Used to distinguish alkynes from alkenes and alkanes. ### Alkynes: Reactions **Chemical Reactions (primarily addition reactions):** 1. **Addition of Dihydrogen (Hydrogenation):** * Alkyne + H₂ $\xrightarrow{\text{Pt/Pd/Ni}}$ Alkene $\rightarrow$ Alkane (complete reduction). * Alkyne + H₂ $\xrightarrow{\text{Lindlar's}}$ Cis-Alkene (partial reduction). * Alkyne + H₂ $\xrightarrow{\text{Na/liquid NH}_3}$ Trans-Alkene (partial reduction). 2. **Addition of Halogens:** * Alkyne + $\text{X}_2 \rightarrow$ Vicinal Dihaloalkene * Vicinal Dihaloalkene + $\text{X}_2 \rightarrow$ Tetrahaloalkane * Example: $\text{CH}_3\text{C}\equiv\text{CH} + \text{Br}_2 \rightarrow \text{CH}_3\text{CBr}=\text{CHBr} \xrightarrow{\text{Br}_2}$ $\text{CH}_3\text{CBr}_2\text{CHBr}_2$ (Decolorizes $\text{Br}_2/\text{CCl}_4$, test for unsaturation). 3. **Addition of Hydrogen Halides (HX):** * Alkyne + HX $\rightarrow$ Vinylic Halide (Markovnikov's rule). * Vinylic Halide + HX $\rightarrow$ Gem-Dihalide (both halogens on same carbon, Markovnikov's rule). * Example: $\text{HC}\equiv\text{CH} + \text{HCl} \rightarrow \text{CH}_2=\text{CHCl}$ (Chloroethene) $\xrightarrow{\text{HCl}}$ $\text{CH}_3\text{CHCl}_2$ (1,1-Dichloroethane). 4. **Addition of Water (Hydration):** * Alkyne + $\text{H}_2\text{O} \xrightarrow{\text{Hg}^{2+}/\text{H}^+}$ Enol $\rightarrow$ Aldehyde/Ketone (isomerization). * Terminal alkynes (except ethyne) give ketones. Ethyne gives ethanal. * Example: $\text{HC}\equiv\text{CH} + \text{H}_2\text{O} \xrightarrow{\text{Hg}^{2+}/\text{H}^+}$ $[\text{CH}_2=\text{CHOH}] \rightarrow \text{CH}_3\text{CHO}$ (Ethyne to Ethanal). 5. **Polymerization:** * **Linear Polymerization:** Ethyne $\rightarrow$ Polyacetylene (conducts electricity). * **Cyclic Polymerization:** Ethyne $\xrightarrow{\text{red hot iron tube, }873\text{K}}$ Benzene. ### Aromatic Hydrocarbons - Also known as **arenes**. - Often possess pleasant odors (hence "aromatic"). - Contain benzene ring (benzenoids) or other aromatic systems (non-benzenoids). - Benzene ring is highly unsaturated but undergoes substitution rather than addition reactions due to its unique stability. **Nomenclature & Isomerism:** - **Monosubstituted Benzene:** Substituent + benzene (e.g., Methylbenzene/Toluene). All 6 H atoms are equivalent. - **Disubstituted Benzene:** * **Ortho (o-):** 1,2 positions. * **Meta (m-):** 1,3 positions. * **Para (p-):** 1,4 positions. - **Polysubstituted Benzene:** Number the ring to give substituents the lowest possible numbers, then list alphabetically. Common names are often preferred (e.g., Xylene for Dimethylbenzene). **Structure of Benzene:** - Molecular formula: $\text{C}_6\text{H}_6$. - **Kekulé Structure:** Proposed cyclic structure with alternating single and double bonds. * Implies two isomeric 1,2-dibromobenzenes, but only one is observed. * Fails to explain unusual stability and preference for substitution. - **Resonance & Stability:** * Benzene is a hybrid of two Kekulé structures (contributing structures). * Actual structure is more stable than any single contributing structure (resonance stabilization energy). * All C-C bond lengths are identical (139 pm), intermediate between single (154 pm) and double (133 pm) bonds. * All carbon atoms are $\text{sp}^2$ hybridized. Each carbon has one unhybridized p orbital perpendicular to the ring plane. * Lateral overlap of these p orbitals forms a delocalized $\pi$-electron cloud above and below the ring. This delocalization is key to its stability. **Aromaticity (Hückel Rule):** For a compound to be aromatic, it must be: 1. **Planar.** 2. **Cyclic.** 3. Have complete **delocalization of $\pi$ electrons.** 4. Possess **(4n+2) $\pi$ electrons** (where n = 0, 1, 2...). * Benzene: n=1, (4*1+2) = 6 $\pi$ electrons. **Examples of Aromatic Compounds:** - Benzene (6$\pi$ e⁻), Naphthalene (10$\pi$ e⁻), Anthracene (14$\pi$ e⁻), Phenanthrene (14$\pi$ e⁻). - Cyclopentadienyl anion (6$\pi$ e⁻), Cycloheptatrienyl cation (6$\pi$ e⁻). ### Aromatic Hydrocarbons: Preparation 1. **Cyclic Polymerization of Ethyne:** * $3\text{HC}\equiv\text{CH} \xrightarrow{\text{red hot iron tube, }873\text{K}}$ Benzene. 2. **Decarboxylation of Aromatic Acids:** * Sodium salt of benzoic acid + Soda lime $\xrightarrow{\Delta}$ Benzene + $\text{Na}_2\text{CO}_3$. * Example: $\text{C}_6\text{H}_5\text{COONa} + \text{NaOH} \xrightarrow{\text{CaO}}$ $\text{C}_6\text{H}_6 + \text{Na}_2\text{CO}_3$. 3. **Reduction of Phenol:** * Phenol + Zn dust $\xrightarrow{\Delta}$ Benzene + ZnO. * Example: $\text{C}_6\text{H}_5\text{OH} + \text{Zn} \rightarrow \text{C}_6\text{H}_6 + \text{ZnO}$. ### Aromatic Hydrocarbons: Properties **Physical Properties:** - Non-polar, colorless liquids/solids with characteristic aroma. - Immiscible with water, miscible with organic solvents. - Burn with sooty flame (high carbon content). **Chemical Properties (Electrophilic Substitution Reactions):** - Benzene's stability leads to substitution rather than addition. - **Mechanism:** 1. **Generation of Electrophile ($\text{E}^+$):** Reagent reacts to form an electrophile. 2. **Formation of Carbocation (Arenium Ion):** $\text{E}^+$ attacks the $\pi$ system, forming a delocalized carbocation intermediate (loses aromaticity). 3. **Removal of Proton:** Proton is removed, restoring aromaticity. 1. **Halogenation:** * Benzene + $\text{X}_2 \xrightarrow{\text{Lewis acid (FeX}_3\text{)}}$ Halobenzene + HX. * Example: $\text{C}_6\text{H}_6 + \text{Cl}_2 \xrightarrow{\text{FeCl}_3}$ $\text{C}_6\text{H}_5\text{Cl} + \text{HCl}$ (Chlorobenzene). 2. **Nitration:** * Benzene + $\text{HNO}_3 \xrightarrow{\text{conc. H}_2\text{SO}_4}$ Nitrobenzene + $\text{H}_2\text{O}$. * Electrophile: Nitronium ion ($\text{NO}_2^+$), generated from $\text{HNO}_3 + 2\text{H}_2\text{SO}_4 \rightarrow \text{NO}_2^+ + \text{H}_3\text{O}^+ + 2\text{HSO}_4^-$. 3. **Sulphonation:** * Benzene + Fuming $\text{H}_2\text{SO}_4 \rightarrow$ Benzenesulphonic acid + $\text{H}_2\text{O}$. * Electrophile: $\text{SO}_3$. 4. **Friedel-Crafts Alkylation:** * Benzene + Alkyl halide $\xrightarrow{\text{Lewis acid (AlCl}_3\text{)}}$ Alkylbenzene + HX. * Example: $\text{C}_6\text{H}_6 + \text{CH}_3\text{Cl} \xrightarrow{\text{AlCl}_3}$ $\text{C}_6\text{H}_5\text{CH}_3 + \text{HCl}$ (Toluene). * May undergo rearrangements if primary carbocations are formed. 5. **Friedel-Crafts Acylation:** * Benzene + Acyl halide/Acid anhydride $\xrightarrow{\text{Lewis acid (AlCl}_3\text{)}}$ Acylbenzene + HX/Acid. * Example: $\text{C}_6\text{H}_6 + \text{CH}_3\text{COCl} \xrightarrow{\text{AlCl}_3}$ $\text{C}_6\text{H}_5\text{COCH}_3 + \text{HCl}$ (Acetophenone). **Directive Influence of Functional Groups (in monosubstituted benzene):** - Groups already present on benzene direct incoming electrophile to specific positions. 1. **Ortho-Para Directing Groups:** Activate the ring (electron-donating) and direct to ortho and para positions. * **Examples:** $\text{-OH}$, $\text{-OR}$, $\text{-NH}_2$, $\text{-NHR}$, $\text{-NR}_2$, $\text{-CH}_3$, $\text{-R}$, halogens ($\text{-F}$, $\text{-Cl}$, $\text{-Br}$, $\text{-I}$). * **Reason:** Electron donation via resonance (or hyperconjugation for alkyl groups) increases electron density at ortho and para positions. Halogens are deactivating but o,p-directing due to resonance. 2. **Meta Directing Groups:** Deactivate the ring (electron-withdrawing) and direct to meta positions. * **Examples:** $\text{-NO}_2$, $\text{-CN}$, $\text{-CHO}$, $\text{-COR}$, $\text{-COOH}$, $\text{-COOR}$, $\text{-SO}_3\text{H}$. * **Reason:** Electron withdrawal via resonance reduces electron density more at ortho and para positions, leaving meta relatively electron-rich. **Additional Reactions:** - **Hydrogenation:** Benzene + 3H₂ $\xrightarrow{\text{Ni}}$ Cyclohexane (under vigorous conditions). - **Addition of Chlorine:** Benzene + 3$\text{Cl}_2 \xrightarrow{\text{uv}}$ Benzene Hexachloride (BHC, gammaxane). - **Combustion:** Benzene + $\text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O}$ (sooty flame). ### Carcinogenicity and Toxicity - **Polynuclear Hydrocarbons:** Hydrocarbons with two or more benzene rings fused together (e.g., 1,2-Benzanthracene, 3-Methylcholanthrene, 1,2,5,6-Dibenzanthracene, 9,10-Dimethyl-1,2-benzanthracene). - **Carcinogenic:** Many are cancer-producing (carcinogenic). - **Formation:** Formed during incomplete combustion of organic materials (tobacco, coal, petroleum). - **Mechanism:** Enter human body, undergo biochemical reactions, damage DNA, and lead to cancer.