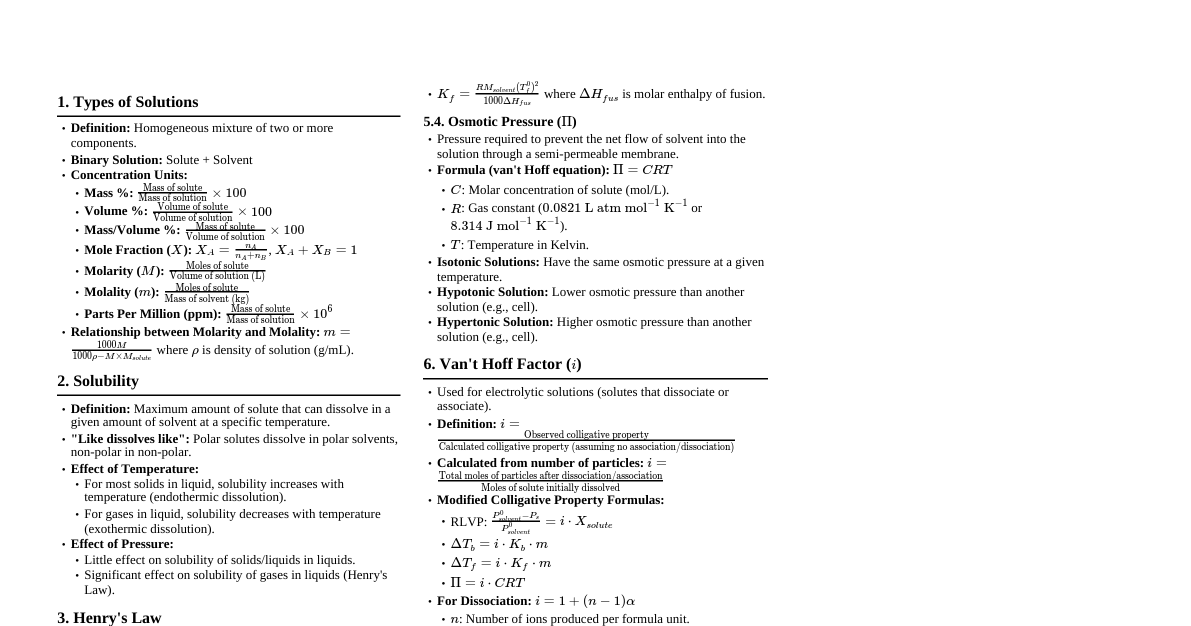
### Solutions: Basics A **solution** is a homogeneous mixture of two or more substances where the components are uniformly distributed at a molecular level. - **Solute:** The substance present in a smaller amount, which dissolves. - **Solvent:** The substance present in a larger amount, which dissolves the solute. Often, water is the "universal solvent" due to its polarity. - **Homogeneous Mixture:** Components are evenly distributed, and individual components cannot be visually distinguished. Examples: Saltwater, air. - **Heterogeneous Mixture:** Components are not evenly distributed and can often be seen separately. Examples: Oil and water, sand in water. #### Types of Solutions by State 1. **Solid in Liquid:** Sugar in water, salt in water. 2. **Liquid in Liquid:** Alcohol in water (e.g., ethanol in water to make alcoholic beverages). 3. **Gas in Gas:** Air (Nitrogen is the solvent, Oxygen, Argon, CO$_2$ are solutes). 4. **Gas in Liquid:** Dissolved oxygen in water (essential for aquatic life), carbon dioxide in soda. 5. **Solid in Solid:** Alloys like brass (zinc in copper). 6. **Liquid in Solid:** Amalgam (mercury in silver, used in dentistry). ### Concentration & Solubility - **Concentration:** Quantifies the amount of solute present in a given amount of solution or solvent. - **Dilute Solution:** Contains a relatively small proportion of solute compared to the solvent. - **Concentrated Solution:** Contains a relatively large proportion of solute. - *Quantitative Expression:* - **Mass by Mass Percentage (% w/w):** $\frac{\text{Mass of solute}}{\text{Mass of solution}} \times 100\%$ - **Mass by Volume Percentage (% w/v):** $\frac{\text{Mass of solute}}{\text{Volume of solution}} \times 100\%$ - **Volume by Volume Percentage (% v/v):** $\frac{\text{Volume of solute}}{\text{Volume of solution}} \times 100\%$ (often used for liquid-liquid solutions) - **Solubility:** The maximum amount of solute that can dissolve in a specific quantity of solvent (e.g., 100 g or 100 mL) at a particular temperature to form a saturated solution. It is a characteristic property of a substance. #### Types of Solutions by Saturation 1. **Unsaturated Solution:** Contains less solute than the maximum amount that can be dissolved at a given temperature. More solute can still be added and dissolved. 2. **Saturated Solution:** Contains the maximum amount of solute that can be dissolved at a given temperature. Any additional solute will remain undissolved and settle at the bottom, creating an equilibrium between dissolved and undissolved solute. 3. **Supersaturated Solution:** Contains more solute than a saturated solution at the same temperature. These are unstable and can be formed by carefully cooling a saturated solution. Disturbing them (e.g., adding a seed crystal) will cause the excess solute to crystallize out. #### Factors Affecting Solubility 1. **Nature of Solute and Solvent ("Like Dissolves Like"):** - Polar solutes tend to dissolve in polar solvents (e.g., salt in water). - Non-polar solutes tend to dissolve in non-polar solvents (e.g., oil in benzene). 2. **Temperature:** - For most **solid solutes in liquid solvents**, solubility generally **increases** with increasing temperature. This is because higher kinetic energy of solvent molecules leads to more frequent and energetic collisions, breaking solute bonds more effectively. - For **gaseous solutes in liquid solvents**, solubility generally **decreases** with increasing temperature. Gases have higher kinetic energy at higher temperatures, making them escape the solvent more easily. (e.g., warm soda goes flat faster). 3. **Pressure:** - For **gaseous solutes in liquid solvents**, solubility generally **increases** with increasing pressure (Henry's Law: $P = k_H C$, where $P$ is partial pressure of gas, $C$ is concentration, $k_H$ is Henry's constant). This forces more gas molecules into solution. (e.g., carbonated drinks are bottled under high CO$_2$ pressure). - Pressure has a **negligible effect** on the solubility of solids and liquids in liquid solvents. #### Activity: Investigating Solubility - **Objective:** To observe the effect of temperature on the solubility of a solid. - **Materials:** Sugar, water, two beakers, stirring rods, hot plate/burner, ice bath. - **Procedure:** 1. Add 100 mL of cold water to one beaker and 100 mL of warm water to another. 2. Add sugar (e.g., 1 teaspoon) to each beaker and stir until dissolved. 3. Continue adding sugar teaspoon by teaspoon to both beakers, stirring well after each addition, until no more sugar dissolves (i.e., a saturated solution is formed and excess sugar settles at the bottom). 4. Compare the amount of sugar dissolved in cold vs. warm water. - **Observation:** More sugar will dissolve in the warm water, demonstrating that solubility of sugar increases with temperature. ### Density: Basics - **Definition:** Density ($\rho$) is an intrinsic physical property of matter, defined as the mass ($m$) per unit volume ($V$) of a substance. $$\rho = \frac{m}{V}$$ - **Units:** - SI Unit: kilograms per cubic meter (kg/m$^3$). - Common laboratory units: grams per milliliter (g/mL) or grams per cubic centimeter (g/cm$^3$). - Conversion: $1 \text{ g/cm}^3 = 1000 \text{ kg/m}^3$. - Density of water at 4°C is approximately 1 g/mL or 1000 kg/m$^3$. #### Factors Affecting Density 1. **Temperature:** - Generally, density **decreases** with an increase in temperature because most substances expand when heated (volume increases while mass remains constant). - **Anomalous Expansion of Water:** Water exhibits unusual behavior. Its density increases as it is cooled from 0°C to 4°C, reaching its maximum density at 4°C. Below 4°C, as it cools further to 0°C and freezes into ice, it expands (volume increases), making ice less dense than liquid water. This is why ice floats, which is crucial for aquatic life in cold climates. 2. **Pressure:** - For **gases**, density **increases** significantly with an increase in pressure because gas molecules are forced closer together, reducing the volume. - For **liquids and solids**, pressure has a **negligible effect** on density due to their incompressible nature (molecules are already very close). #### Floating and Sinking: Archimedes' Principle - **Archimedes' Principle:** States that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object. - **Buoyant Force ($F_B$):** The upward force exerted by a fluid that opposes the weight of an immersed object. $F_B = \rho_{fluid} \times V_{displaced} \times g$. - **Conditions for Floating/Sinking:** - An object **floats** if its average density is less than the density of the fluid it is in ($\rho_{object} \rho_{fluid}$). The buoyant force is less than the object's weight. - An object will be **suspended** (neither float nor sink, remaining at any level) if its average density is equal to the density of the fluid ($\rho_{object} = \rho_{fluid}$). - **Relative Density (Specific Gravity):** The ratio of the density of a substance to the density of a reference substance (usually water at 4°C). It is a unitless quantity and indicates how many times denser or lighter a substance is compared to water. $$\text{Relative Density} = \frac{\text{Density of substance}}{\text{Density of water at } 4^\circ\text{C}}$$ - If Relative Density 1, the substance sinks in water. #### Activity: Density Column - **Objective:** To observe density differences between various liquids. - **Materials:** Tall glass, honey, corn syrup, dish soap, water (colored with food coloring), vegetable oil, rubbing alcohol (colored), small objects (grape, cherry tomato, plastic bead, coin). - **Procedure:** 1. Carefully pour equal amounts of each liquid into the glass, one by one, in the following order: honey, corn syrup, dish soap, colored water, vegetable oil, colored rubbing alcohol. Pour slowly down the side of the glass to prevent mixing. 2. Observe the layers formed. 3. Gently drop in the small objects and observe where they settle in the column. - **Observation:** The liquids will layer according to their densities (densest at the bottom, least dense at the top). The objects will settle at the layer where their density is approximately equal to or less than the liquid they are floating on. ### Measuring Mass and Volume #### Mass Measurement - **Mass:** A measure of the amount of matter in an object. It is a fundamental property and remains constant regardless of location (unlike weight). - **Instrument:** Various types of balances: - **Beam Balances:** (e.g., two-pan balance, triple beam balance) work by comparing an unknown mass to known masses. - **Electronic/Digital Balances:** Provide a direct numerical reading of mass and are commonly used in modern labs. - **Units:** gram (g), kilogram (kg), milligram (mg). - **Precision:** Balances can measure mass with varying degrees of precision (e.g., to 0.01 g or 0.001 g). #### Volume Measurement - **Volume:** The amount of three-dimensional space occupied by a substance or object. - **Units:** cubic meter (m$^3$), liter (L), milliliter (mL), cubic centimeter (cm$^3$). - Key Conversions: $1 \text{ L} = 1 \text{ dm}^3$; $1 \text{ mL} = 1 \text{ cm}^3$. - **Instruments for Liquids:** - **Measuring Cylinder (Graduated Cylinder):** Used for approximate measurements of liquid volumes. Marked with a scale. - **Meniscus:** The curved surface of a liquid in a container. For water and aqueous solutions, the meniscus curves downwards (concave). Readings should be taken at the bottom of the meniscus at eye level to avoid parallax error. For mercury, the meniscus is convex, and readings are taken at the top. - **Burette:** Used for precise delivery of variable volumes of liquid, especially in titrations. Has a stopcock to control flow. Readings are taken before and after liquid delivery. - **Pipette:** Used for precise measurement and transfer of fixed volumes of liquid (e.g., 10 mL, 25 mL). Volumetric pipettes are highly accurate. - **Beakers and Conical Flasks (Erlenmeyer Flasks):** Used for holding, mixing, and heating liquids, but their volume markings are approximate and not suitable for precise measurements. - **Methods for Solids:** - **For Regular Shaped Solids:** Volume is calculated using geometric formulas. - Cube: $V = \text{side}^3$ - Cuboid: $V = \text{length} \times \text{width} \times \text{height}$ - Cylinder: $V = \pi \times \text{radius}^2 \times \text{height}$ - Sphere: $V = \frac{4}{3} \pi \times \text{radius}^3$ - **For Irregular Shaped Solids (Water Displacement Method):** Based on Archimedes' Principle. 1. Add a known volume of water ($V_1$) to a measuring cylinder or overflow can. 2. Carefully immerse the irregular solid into the water. Ensure it is fully submerged and no air bubbles are trapped. 3. Read the new volume of water + solid ($V_2$). 4. The volume of the solid is the difference: $V_{solid} = V_2 - V_1$. 5. *Using an Overflow Can:* If the object is too large for a measuring cylinder, submerge it in an overflow can filled to the brim. Collect the displaced water in a measuring cylinder, and its volume is the volume of the solid. #### Activity: Determining Density of an Irregular Object - **Objective:** To determine the density of an irregular-shaped object (e.g., a small rock). - **Materials:** Small rock, electronic balance, measuring cylinder, water. - **Procedure:** 1. **Measure Mass:** Place the rock on the electronic balance and record its mass ($m$). 2. **Measure Initial Water Volume:** Pour enough water into the measuring cylinder to submerge the rock, and record the initial volume ($V_1$). 3. **Submerge Object:** Gently lower the rock into the water in the measuring cylinder, ensuring no water splashes out and no air bubbles are clinging to the rock. 4. **Measure Final Water Volume:** Record the new volume ($V_2$). 5. **Calculate Volume of Rock:** $V_{rock} = V_2 - V_1$. 6. **Calculate Density:** $\rho_{rock} = \frac{m_{rock}}{V_{rock}}$. - **Extension:** Compare the calculated density with known densities to identify the type of rock or predict if it would float or sink in other liquids.