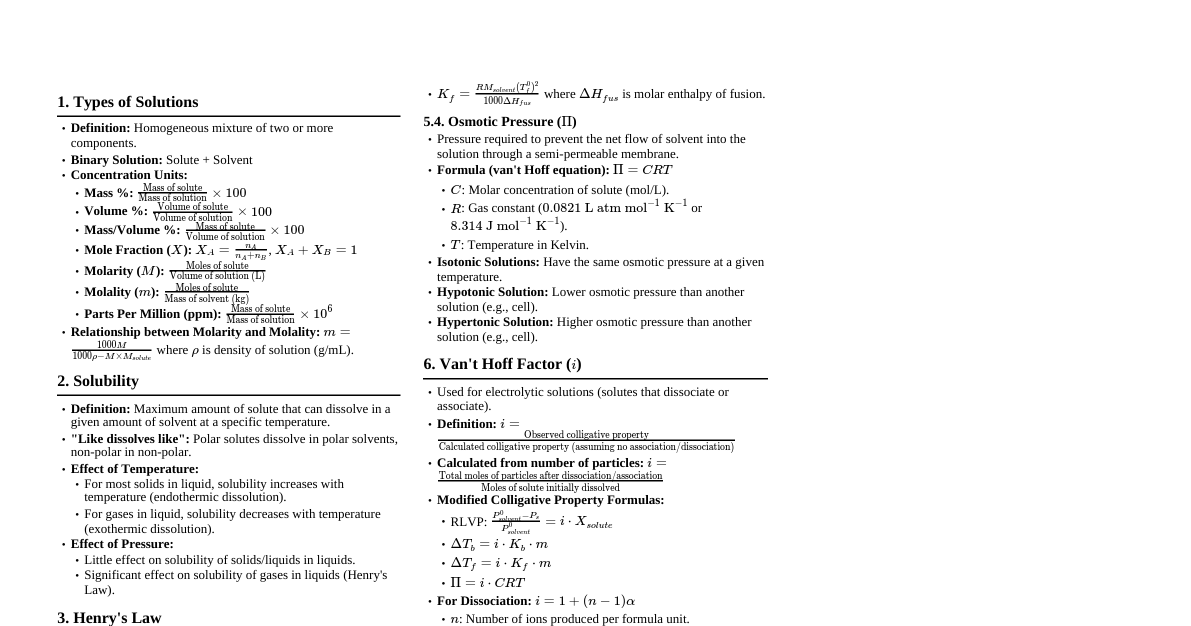
1. Types of Solutions Solution: Homogeneous mixture of two or more components. Binary Solution: Contains two components (solute and solvent). Solvent: Component present in larger quantity, determines physical state. Solute: Component present in smaller quantity. Types based on physical state: Gas in Gas (Air) Gas in Liquid (Aerated drinks) Gas in Solid (Hydrogen in Palladium) Liquid in Gas (Fog) Liquid in Liquid (Alcohol in water) Liquid in Solid (Amalgam) Solid in Gas (Smoke) Solid in Liquid (Sugar in water) Solid in Solid (Alloys) 2. Expressing Concentration of Solutions Mass Percentage (w/w): $\text{Mass %} = \frac{\text{Mass of solute}}{\text{Mass of solution}} \times 100$ Volume Percentage (v/v): $\text{Volume %} = \frac{\text{Volume of solute}}{\text{Volume of solution}} \times 100$ Mass by Volume Percentage (w/v): $\text{Mass/Volume %} = \frac{\text{Mass of solute}}{\text{Volume of solution}} \times 100$ Parts per Million (ppm): Used for very dilute solutions. $\text{ppm} = \frac{\text{Mass of solute}}{\text{Mass of solution}} \times 10^6$ Mole Fraction ($\chi$): Ratio of moles of one component to total moles. $\chi_A = \frac{n_A}{n_A + n_B}$, $\chi_B = \frac{n_B}{n_A + n_B}$, $\chi_A + \chi_B = 1$ Molarity (M): Moles of solute per litre of solution. $M = \frac{\text{Moles of solute}}{\text{Volume of solution (L)}}$ Molality (m): Moles of solute per kilogram of solvent. $m = \frac{\text{Moles of solute}}{\text{Mass of solvent (kg)}}$ Normality (N): Gram equivalents of solute per litre of solution. $N = \frac{\text{Gram equivalents of solute}}{\text{Volume of solution (L)}}$ $\text{Gram equivalents} = \frac{\text{Mass of solute}}{\text{Equivalent mass}}$ 3. Solubility Definition: Maximum amount of solute that can dissolve in a specified amount of solvent at a specified temperature. Saturated Solution: Contains maximum amount of solute that can be dissolved. Unsaturated Solution: Contains less than maximum amount of solute. Supersaturated Solution: Contains more solute than required to saturate it. (Unstable) 3.1. Solubility of Solids in Liquids "Like dissolves like": Polar solutes dissolve in polar solvents, non-polar in non-polar. Effect of Temperature: Endothermic dissolution: Solubility increases with temperature. Exothermic dissolution: Solubility decreases with temperature. Effect of Pressure: No significant effect on solid solubility. 3.2. Solubility of Gases in Liquids Effect of Temperature: Solubility of gases in liquids decreases with increasing temperature. Effect of Pressure: Solubility of gases in liquids increases with increasing pressure. 4. Henry's Law States that the partial pressure of a gas in vapor phase ($p$) is proportional to the mole fraction of the gas ($\chi$) in the solution. $p = K_H \chi$ where $K_H$ is Henry's Law constant. Higher $K_H$ value indicates lower solubility of the gas. Applications: Soft drinks (CO$_2$ dissolved under high pressure). Deep-sea diving (N$_2$ solubility increases, causing "bends"). High altitude (low $\text{O}_2$ partial pressure, causing anoxia). 5. Vapour Pressure of Liquid Solutions Vapour Pressure: Pressure exerted by the vapours in equilibrium with the liquid at a given temperature. Factors affecting Vapour Pressure: Nature of liquid, temperature. 5.1. Raoult's Law For a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction. $p_A = p_A^0 \chi_A$ $p_B = p_B^0 \chi_B$ where $p_A^0$ and $p_B^0$ are vapour pressures of pure components A and B. Dalton's Law of Partial Pressures: Total vapour pressure $P_{total} = p_A + p_B = p_A^0 \chi_A + p_B^0 \chi_B$. For a solution of non-volatile solute in a volatile solvent: $P_{solution} = p_A = p_A^0 \chi_A$ The vapour pressure of the solution is solely due to the solvent. 6. Ideal and Non-Ideal Solutions Ideal Solutions: Obey Raoult's Law over the entire range of concentrations. $\Delta H_{mix} = 0$ (no heat absorbed/released on mixing). $\Delta V_{mix} = 0$ (no volume change on mixing). Intermolecular forces A-B are similar to A-A and B-B. E.g., Benzene + Toluene, n-Hexane + n-Heptane. Non-Ideal Solutions: Deviate from Raoult's Law. Positive Deviation: $P_{total} > (p_A^0 \chi_A + p_B^0 \chi_B)$ $\Delta H_{mix} > 0$ (endothermic) $\Delta V_{mix} > 0$ (volume increases) A-B forces weaker than A-A and B-B. E.g., Ethanol + Acetone, CCl$_4$ + Toluene. Negative Deviation: $P_{total} $\Delta H_{mix} $\Delta V_{mix} A-B forces stronger than A-A and B-B. E.g., Chloroform + Acetone, Nitric acid + Water. 7. Azeotropes Binary mixtures having the same composition in liquid and vapour phases, and boil at a constant temperature. Cannot be separated by fractional distillation. Minimum Boiling Azeotropes: Formed by solutions showing large positive deviation from Raoult's Law (e.g., Ethanol-Water). Maximum Boiling Azeotropes: Formed by solutions showing large negative deviation from Raoult's Law (e.g., Nitric acid-Water). 8. Colligative Properties Properties of dilute solutions that depend only on the number of solute particles, not on their nature. Applicable for non-volatile solutes. 8.1. Relative Lowering of Vapour Pressure (RLVP) For a solution of non-volatile solute: $\frac{p_A^0 - p_A}{p_A^0} = \chi_B = \frac{n_B}{n_A + n_B}$ For very dilute solutions: $\frac{p_A^0 - p_A}{p_A^0} \approx \frac{n_B}{n_A}$ RLVP is equal to the mole fraction of the solute. 8.2. Elevation in Boiling Point ($\Delta T_b$) Boiling point of a solution is higher than that of the pure solvent. $\Delta T_b = T_b - T_b^0$ $\Delta T_b = K_b m$ where $K_b$ is ebullioscopic constant (molal elevation constant). $K_b = \frac{R M_1 (T_b^0)^2}{1000 \Delta_{vap} H}$ 8.3. Depression in Freezing Point ($\Delta T_f$) Freezing point of a solution is lower than that of the pure solvent. $\Delta T_f = T_f^0 - T_f$ $\Delta T_f = K_f m$ where $K_f$ is cryoscopic constant (molal depression constant). $K_f = \frac{R M_1 (T_f^0)^2}{1000 \Delta_{fus} H}$ 8.4. Osmosis and Osmotic Pressure ($\Pi$) Osmosis: Spontaneous net movement of solvent molecules through a semi-permeable membrane from a region of higher solvent concentration to a region of lower solvent concentration. Osmotic Pressure ($\Pi$): The excess pressure that must be applied to a solution to prevent osmosis (i.e., to stop the net flow of solvent into the solution through a semi-permeable membrane). $\Pi = CRT$ where $C$ is molar concentration, $R$ is gas constant, $T$ is temperature in Kelvin. Isotonic Solutions: Have the same osmotic pressure (e.g., 0.9% NaCl solution with blood cells). Hypotonic Solution: Lower osmotic pressure than another solution; cells swell. Hypertonic Solution: Higher osmotic pressure than another solution; cells shrink. Reverse Osmosis: Application of pressure greater than osmotic pressure to force solvent from solution through a semi-permeable membrane into pure solvent (used in desalination). 9. Abnormal Molar Masses When solute undergoes association or dissociation in solution, the number of particles changes, leading to abnormal values for colligative properties and thus abnormal molar masses. Van't Hoff Factor ($i$): $i = \frac{\text{Normal molar mass}}{\text{Observed (abnormal) molar mass}}$ $i = \frac{\text{Observed colligative property}}{\text{Calculated colligative property (assuming no association/dissociation)}}$ $i = \frac{\text{Total number of moles of particles after association/dissociation}}{\text{Number of moles of particles before association/dissociation}}$ Modified Colligative Property Equations: RLVP: $\frac{p_A^0 - p_A}{p_A^0} = i \chi_B$ Elevation in BP: $\Delta T_b = i K_b m$ Depression in FP: $\Delta T_f = i K_f m$ Osmotic Pressure: $\Pi = i C R T$ For dissociation: $i > 1$ (e.g., NaCl $\rightarrow$ Na$^+$ + Cl$^-$, $i \approx 2$) For association: $i