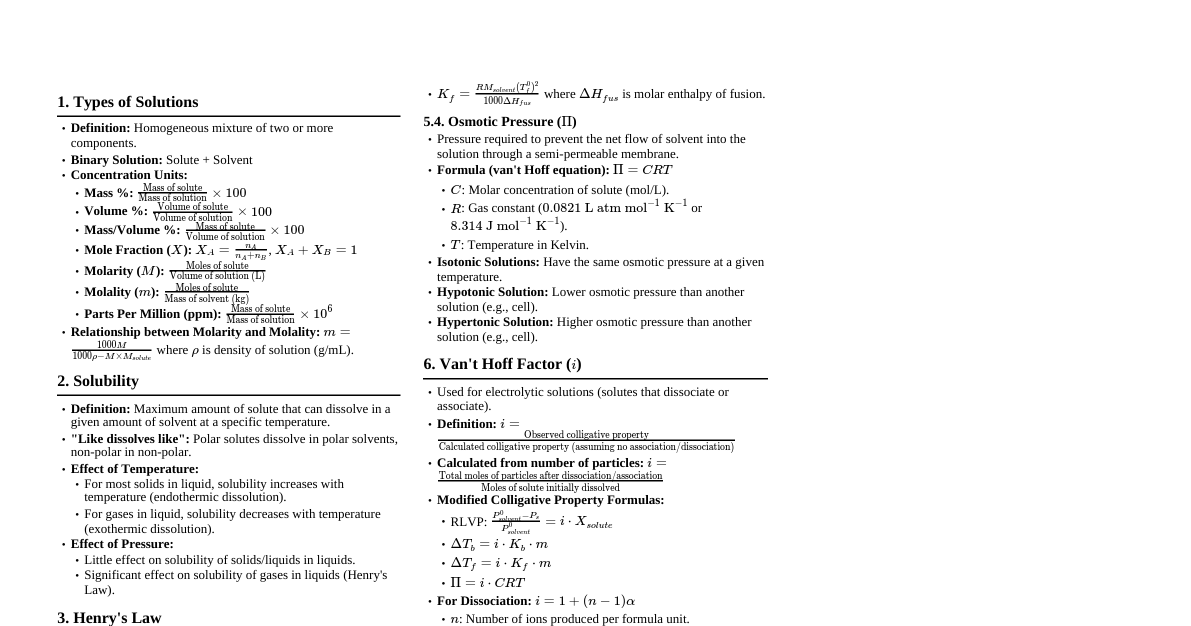
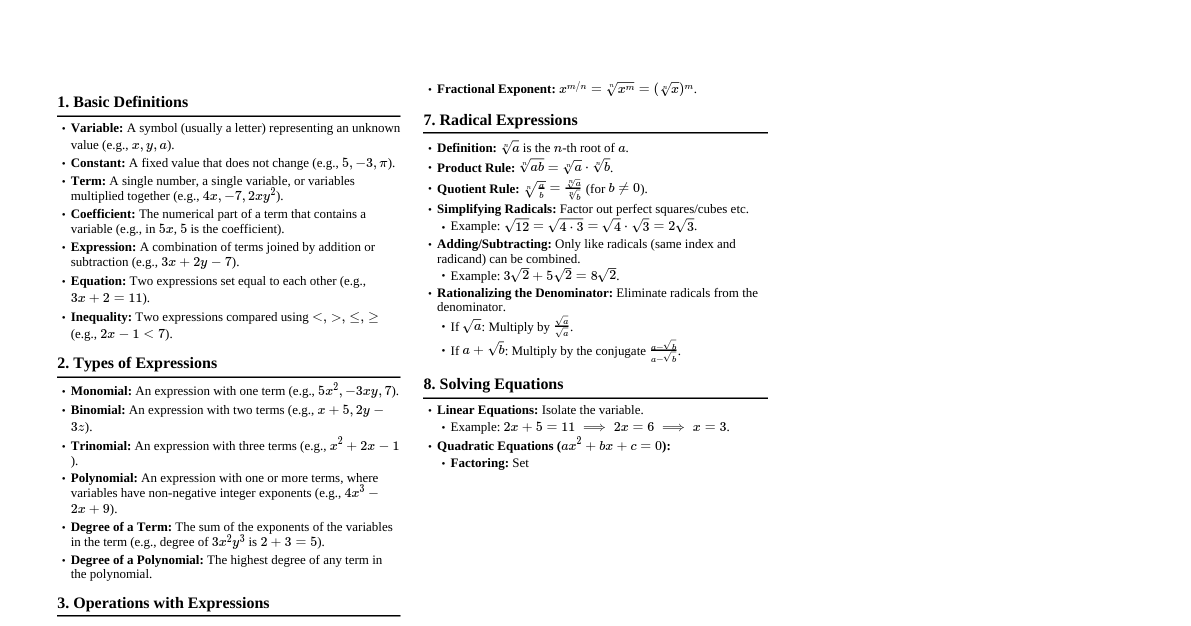
1. Basic Definitions Solution: Homogeneous mixture of two or more substances. Solute: Substance dissolved in a solvent (usually present in smaller amount). Solvent: Substance that dissolves the solute (usually present in larger amount). Aqueous Solution: Solution where water is the solvent. Solubility: Maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. Saturated Solution: Contains the maximum amount of dissolved solute. Unsaturated Solution: Contains less than the maximum amount of dissolved solute. Supersaturated Solution: Contains more than the maximum amount of dissolved solute (unstable). 2. Concentration Units Molarity (M): Moles of solute per liter of solution. $$M = \frac{\text{moles of solute}}{\text{liters of solution}}$$ Molality (m): Moles of solute per kilogram of solvent. $$m = \frac{\text{moles of solute}}{\text{kilograms of solvent}}$$ Mole Fraction ($\chi$): Moles of component divided by total moles of all components. $$\chi_A = \frac{n_A}{n_{\text{total}}}$$ Mass Percent (% w/w): Mass of solute divided by total mass of solution, times 100. $$\text{Mass Percent} = \frac{\text{mass of solute}}{\text{mass of solution}} \times 100\%$$ Volume Percent (% v/v): Volume of solute divided by total volume of solution, times 100. $$\text{Volume Percent} = \frac{\text{volume of solute}}{\text{volume of solution}} \times 100\%$$ Parts per Million (ppm): Mass of solute divided by total mass of solution, times $10^6$. $$\text{ppm} = \frac{\text{mass of solute}}{\text{mass of solution}} \times 10^6$$ 3. Factors Affecting Solubility "Like Dissolves Like": Polar solvents dissolve polar solutes; nonpolar solvents dissolve nonpolar solutes. Temperature: For most solids, solubility increases with increasing temperature. For most gases, solubility decreases with increasing temperature. Pressure (for gases): Henry's Law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the solution. $$C = kP$$ Where $C$ is solubility, $k$ is Henry's Law constant, $P$ is partial pressure. 4. Colligative Properties Properties of solutions that depend on the number of solute particles, not on their identity. Vapor Pressure Lowering (Raoult's Law): The vapor pressure of a solvent above a solution is lower than that of the pure solvent. $$P_A = \chi_A P_A^\circ$$ Where $P_A$ is vapor pressure of solvent in solution, $\chi_A$ is mole fraction of solvent, $P_A^\circ$ is vapor pressure of pure solvent. For non-volatile solute: $\Delta P = \chi_B P_A^\circ$ Boiling Point Elevation: The boiling point of a solution is higher than that of the pure solvent. $$\Delta T_b = i K_b m$$ Where $\Delta T_b$ is boiling point elevation, $i$ is van 't Hoff factor, $K_b$ is molal boiling point elevation constant, $m$ is molality. Freezing Point Depression: The freezing point of a solution is lower than that of the pure solvent. $$\Delta T_f = i K_f m$$ Where $\Delta T_f$ is freezing point depression, $i$ is van 't Hoff factor, $K_f$ is molal freezing point depression constant, $m$ is molality. Osmotic Pressure ($\Pi$): The pressure required to stop osmosis across a semipermeable membrane. $$\Pi = i MRT$$ Where $\Pi$ is osmotic pressure, $i$ is van 't Hoff factor, $M$ is molarity, $R$ is ideal gas constant ($0.08206 \text{ L atm mol}^{-1} \text{ K}^{-1}$), $T$ is temperature in Kelvin. van 't Hoff Factor ($i$): Number of particles a solute dissociates into in solution. For non-electrolytes (e.g., sugar): $i=1$ For strong electrolytes (e.g., NaCl $\rightarrow$ Na$^+$ + Cl$^-$): $i=2$ For weak electrolytes: $1 5. Types of Solutions Ideal Solution: Obeys Raoult's Law over the entire range of concentrations. No enthalpy change on mixing, and no volume change on mixing. Non-Ideal Solution: Deviates from Raoult's Law. Positive Deviation: Vapor pressure higher than predicted; weaker solute-solvent interactions. Negative Deviation: Vapor pressure lower than predicted; stronger solute-solvent interactions. Electrolyte Solution: Contains ions and conducts electricity. Strong Electrolyte: Dissociates completely (e.g., strong acids, strong bases, soluble salts). Weak Electrolyte: Dissociates partially (e.g., weak acids, weak bases). Non-Electrolyte: Does not dissociate into ions (e.g., sugar, alcohol). 6. Dilution Adding more solvent to a solution to decrease its concentration. $$M_1 V_1 = M_2 V_2$$ Where $M_1, V_1$ are initial molarity and volume, and $M_2, V_2$ are final molarity and volume. 7. Solution Stoichiometry Using concentration units in chemical reactions. Moles of solute = Molarity $\times$ Volume (in Liters) Can use mole ratios from balanced chemical equations to relate amounts of reactants and products in solution.