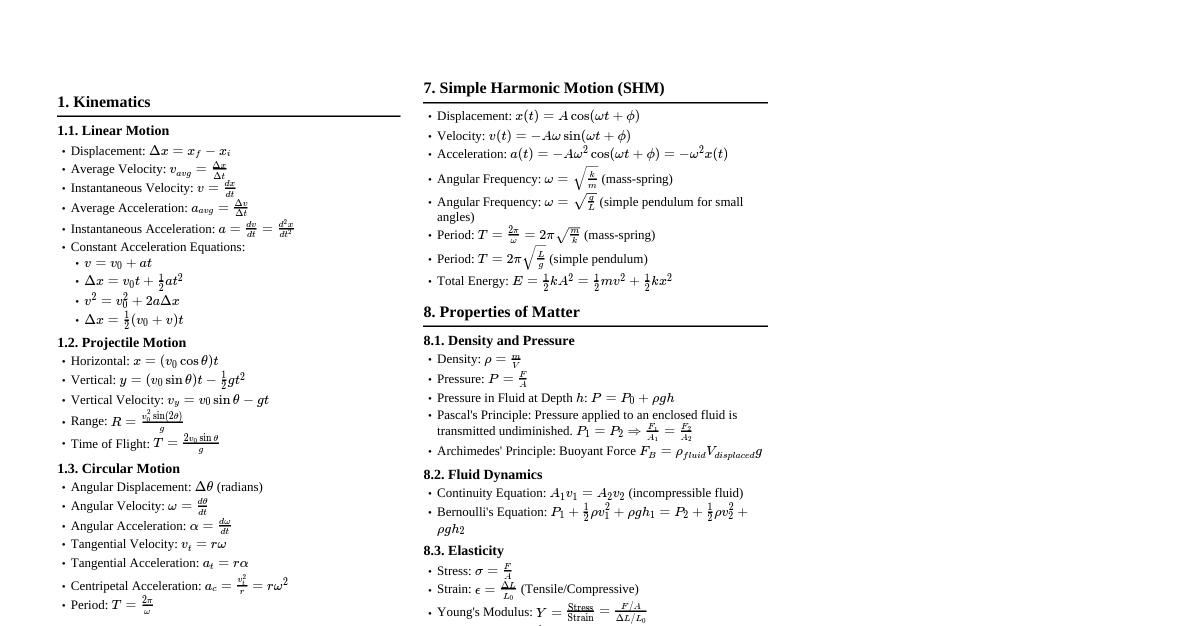
1. Introduction to Heat & Temperature Heat: Form of energy transferred between systems due to temperature difference. Temperature: Measure of hotness or coldness of a body. SI Unit of Heat: Joule (J) SI Unit of Temperature: Kelvin (K), also commonly used Celsius ($^\circ$C). 2. Measurement of Temperature Thermometer: Device used to measure temperature, based on physical properties changing with temperature (e.g., volume of liquid). Temperature Scales: Celsius Scale: Ice point $0^\circ$C, Steam point $100^\circ$C. Fahrenheit Scale: Ice point $32^\circ$F, Steam point $212^\circ$F. Conversion between Scales: $\frac{t_F - 32}{180} = \frac{t_C}{100}$ 3. Ideal-Gas Equation & Absolute Temperature Ideal Gas Law: For low-density gases, $PV/T = \text{constant}$. $PV = \mu RT$ Where $\mu$ is number of moles, $R = 8.31 \, \text{J mol}^{-1} \text{K}^{-1}$ (universal gas constant). Absolute Zero: The theoretical minimum temperature where an ideal gas would have zero pressure, found to be $-273.15^\circ$C. Kelvin Scale: Absolute temperature scale. $0 \, \text{K} = -273.15^\circ$C. $T (\text{K}) = t_C (\text{^\circ C}) + 273.15$ 4. Thermal Expansion Definition: Increase in dimensions of a body due to increase in temperature. Types of Expansion: Linear Expansion: Change in length. $\frac{\Delta l}{l} = \alpha_l \Delta T$ Where $\alpha_l$ is the coefficient of linear expansion. Area Expansion: Change in area. $\frac{\Delta A}{A} = \alpha_A \Delta T \approx 2 \alpha_l \Delta T$ Volume Expansion: Change in volume. $\frac{\Delta V}{V} = \alpha_V \Delta T \approx 3 \alpha_l \Delta T$ Where $\alpha_V$ is the coefficient of volume expansion. Thermal Stress: Stress developed in a material when thermal expansion/contraction is prevented. Thermal Stress $= Y \frac{\Delta l}{l} = Y \alpha_l \Delta T$ Where $Y$ is Young's modulus. Anomalous Expansion of Water: Water contracts from $0^\circ$C to $4^\circ$C, then expands above $4^\circ$C. Maximum density at $4^\circ$C. 5. Specific Heat Capacity Heat Capacity ($S$): Amount of heat required to change the temperature of a substance. $S = \frac{\Delta Q}{\Delta T}$ Specific Heat Capacity ($s$): Heat required per unit mass to change temperature by one unit. $s = \frac{1}{m} \frac{\Delta Q}{\Delta T}$ SI Unit: $\text{J kg}^{-1} \text{K}^{-1}$. Molar Specific Heat Capacity ($C$): Heat required per mole to change temperature by one unit. $C = \frac{1}{\mu} \frac{\Delta Q}{\Delta T}$ SI Unit: $\text{J mol}^{-1} \text{K}^{-1}$. For Gases: $C_P$: Molar specific heat at constant pressure. $C_V$: Molar specific heat at constant volume. 6. Calorimetry Principle: In an isolated system, heat lost by hotter body = heat gained by colder body. Calorimeter: Device for heat measurement, designed to minimize heat exchange with surroundings. 7. Change of State Melting/Fusion: Solid to liquid. Freezing: Liquid to solid. Boiling/Vaporisation: Liquid to gas. Condensation: Gas to liquid. Sublimation: Solid to gas (or vice versa) without passing through liquid state. Melting Point: Temperature at which solid and liquid phases coexist in thermal equilibrium. Boiling Point: Temperature at which liquid and vapour phases coexist in thermal equilibrium. Effect of Pressure on Melting/Boiling Points: Melting point decreases with increased pressure (e.g., ice). Boiling point increases with increased pressure. Regelation: Phenomenon where a substance (like ice) melts under pressure and refreezes when pressure is removed. Triple Point: Specific temperature and pressure at which all three phases (solid, liquid, gas) of a substance coexist in thermal equilibrium. (e.g., water: $273.16 \, \text{K}$ and $6.11 \times 10^{-3} \, \text{Pa}$). Latent Heat ($L$): Amount of heat per unit mass transferred during a change of state at constant temperature. $Q = mL$ SI Unit: $\text{J kg}^{-1}$. Latent Heat of Fusion ($L_f$): For solid-liquid transition. Latent Heat of Vaporisation ($L_v$): For liquid-gas transition. 8. Heat Transfer Conduction: Heat transfer through molecular collisions without actual flow of matter. Occurs mainly in solids. Rate of heat flow ($H$) $= \frac{\Delta Q}{\Delta t} = \frac{KA(T_C - T_D)}{L}$ Where $K$ is thermal conductivity, $A$ is cross-sectional area, $(T_C - T_D)$ is temperature difference, $L$ is length. SI Unit of $K$: $\text{J s}^{-1} \text{m}^{-1} \text{K}^{-1}$ or $\text{W m}^{-1} \text{K}^{-1}$. Convection: Heat transfer by actual movement of fluid (liquid or gas). Natural Convection: Driven by density differences (buoyancy). Forced Convection: Fluid movement aided by external means (e.g., pump). Radiation: Heat transfer via electromagnetic waves, requires no medium. Blackbody Radiation: Radiation emitted by an ideal blackbody, which absorbs all incident radiation. Wien's Displacement Law: Relates peak wavelength of emitted radiation to temperature. $\lambda_m T = \text{constant}$ Where $\text{constant} = 2.9 \times 10^{-3} \, \text{m K}$. Stefan-Boltzmann Law: Total energy radiated per unit time by a blackbody. $H = A \sigma T^4$ Where $\sigma = 5.67 \times 10^{-8} \, \text{W m}^{-2} \text{K}^{-4}$ (Stefan-Boltzmann constant). For a real body with emissivity ($e$): $H = e A \sigma T^4$ Net rate of heat loss to surroundings at temperature $T_s$: $H = e A \sigma (T^4 - T_s^4)$ 9. Newton's Law of Cooling Statement: The rate of loss of heat of a body is directly proportional to the temperature difference between the body and its surroundings, for small temperature differences. $\frac{dQ}{dt} = -k(T_2 - T_1)$ Where $T_2$ is body temperature, $T_1$ is surrounding temperature, $k$ is a positive constant. This can also be expressed as: $ms \frac{dT_2}{dt} = -k(T_2 - T_1)$