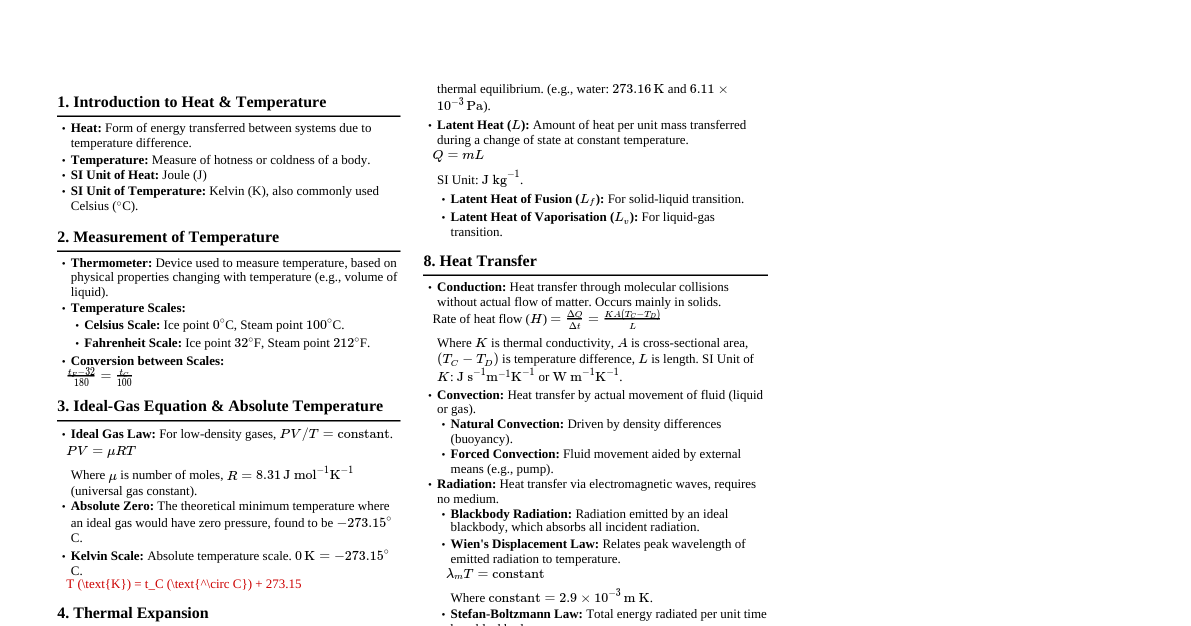
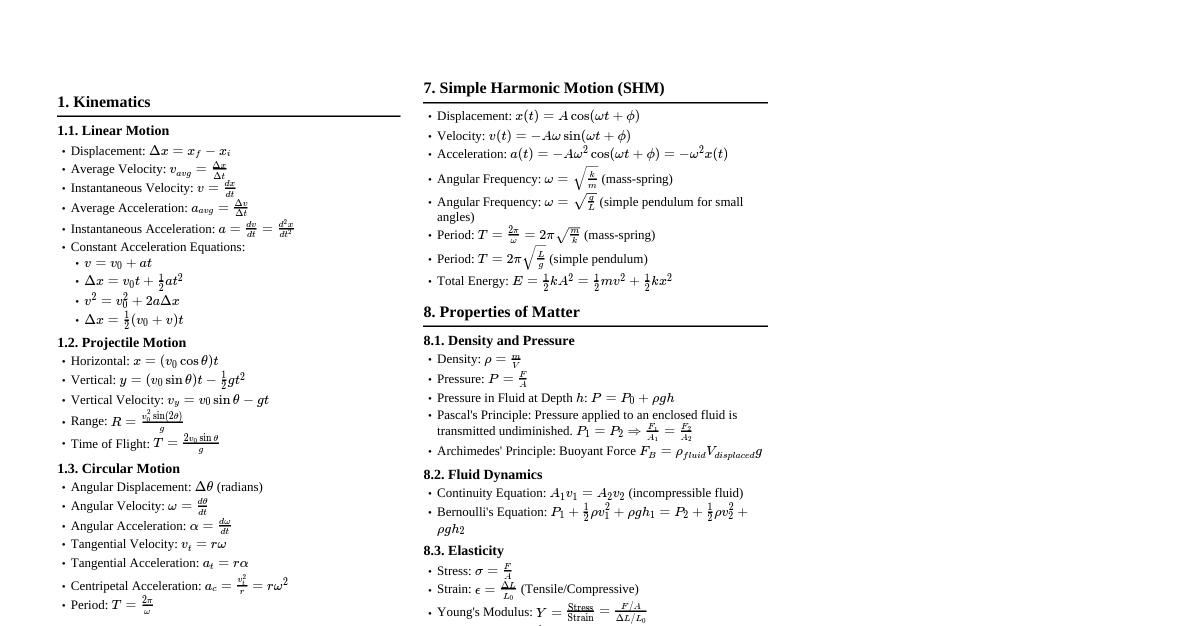
1. Temperature Scales Celsius to Fahrenheit: $F = \frac{9}{5}C + 32$ Fahrenheit to Celsius: $C = \frac{5}{9}(F - 32)$ Celsius to Kelvin: $K = C + 273.15$ Kelvin to Celsius: $C = K - 273.15$ Relationship between scales: $\frac{C}{5} = \frac{F - 32}{9} = \frac{K - 273.15}{5}$ 2. Thermal Expansion 2.1. Linear Expansion Change in length: $\Delta L = L_0 \alpha \Delta T$ Final length: $L = L_0 (1 + \alpha \Delta T)$ $\alpha$: Coefficient of linear expansion (unit: $K^{-1}$ or $^\circ C^{-1}$) 2.2. Area (Superficial) Expansion Change in area: $\Delta A = A_0 \beta \Delta T$ Final area: $A = A_0 (1 + \beta \Delta T)$ $\beta$: Coefficient of area expansion (unit: $K^{-1}$ or $^\circ C^{-1}$) Relation to $\alpha$: $\beta \approx 2\alpha$ 2.3. Volume (Cubical) Expansion Change in volume: $\Delta V = V_0 \gamma \Delta T$ Final volume: $V = V_0 (1 + \gamma \Delta T)$ $\gamma$: Coefficient of volume expansion (unit: $K^{-1}$ or $^\circ C^{-1}$) Relation to $\alpha$: $\gamma \approx 3\alpha$ 2.4. Anomalous Expansion of Water Water contracts from $0^\circ C$ to $4^\circ C$, then expands above $4^\circ C$. Density is maximum at $4^\circ C$. 3. Heat Transfer 3.1. Conduction Rate of heat flow: $\frac{dQ}{dt} = -KA \frac{dT}{dx}$ (Fourier's Law) For steady state through a slab: $\frac{dQ}{dt} = \frac{KA(T_1 - T_2)}{L}$ $K$: Thermal conductivity (unit: $W m^{-1} K^{-1}$) $A$: Cross-sectional area $L$: Thickness Thermal resistance: $R = \frac{L}{KA}$ Series combination: $R_{eq} = R_1 + R_2 + ...$ Parallel combination: $\frac{1}{R_{eq}} = \frac{1}{R_1} + \frac{1}{R_2} + ...$ 3.2. Convection Heat transfer by movement of fluid particles. No specific formula for general cases in this context. 3.3. Radiation Stefan-Boltzmann Law: Power radiated $P = \sigma A e T^4$ $\sigma$: Stefan-Boltzmann constant ($5.67 \times 10^{-8} W m^{-2} K^{-4}$) $A$: Surface area $e$: Emissivity (for a black body, $e=1$) $T$: Absolute temperature of the body (in Kelvin) Net power radiated (body at $T$, surroundings at $T_0$): $P_{net} = \sigma A e (T^4 - T_0^4)$ Wien's Displacement Law: $\lambda_m T = b$ $\lambda_m$: Wavelength corresponding to maximum emission $b$: Wien's constant ($2.898 \times 10^{-3} m K$) 4. Specific Heat Capacity Heat absorbed/released: $Q = mc\Delta T$ $m$: Mass of substance $c$: Specific heat capacity (unit: $J kg^{-1} K^{-1}$ or $J kg^{-1} ^\circ C^{-1}$) $\Delta T$: Change in temperature Molar specific heat capacity: $C = Mc$, where $M$ is molar mass. Water's specific heat capacity: $c_{water} = 4186 J kg^{-1} ^\circ C^{-1}$ or $1 cal g^{-1} ^\circ C^{-1}$ 5. Latent Heat Heat for phase change: $Q = mL$ $m$: Mass of substance $L$: Latent heat (unit: $J kg^{-1}$) Latent heat of fusion ($L_f$): For melting/freezing. Latent heat of vaporization ($L_v$): For boiling/condensation. Latent heat of fusion of ice: $L_f = 3.34 \times 10^5 J kg^{-1}$ Latent heat of vaporization of water: $L_v = 2.26 \times 10^6 J kg^{-1}$ 6. Calorimetry (Principle of Heat Exchange) Heat lost = Heat gained Applicable when different substances at different temperatures are mixed in an isolated system. $m_1 c_1 (T_1 - T_{final}) = m_2 c_2 (T_{final} - T_2)$ (assuming $T_1 > T_2$) 7. Newton's Law of Cooling Rate of cooling: $\frac{dT}{dt} = -k(T - T_s)$ $T$: Temperature of the body $T_s$: Temperature of the surroundings $k$: Positive constant depending on surface area, emissivity, specific heat, and mass of the body. Applicable when the temperature difference between the body and surroundings is small.