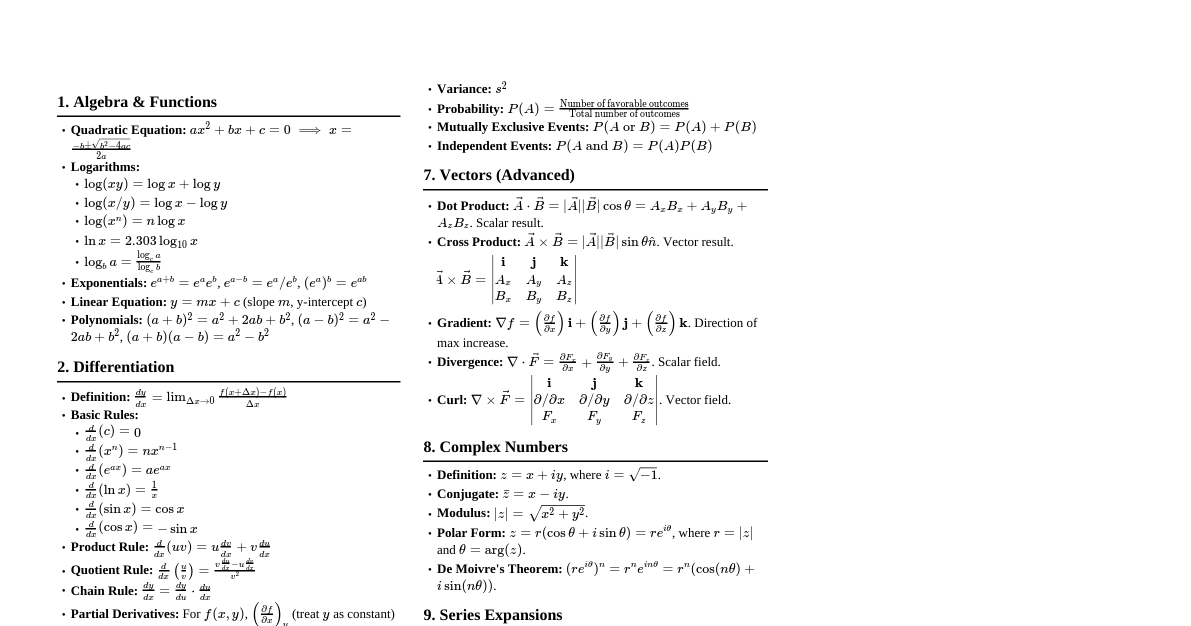
Introduction to Chemistry Definition: Chemistry is the branch of science that deals with the preparation, properties, structure, and reactions of matter. Branches: Inorganic, Organic, Physical, Analytical, Polymer, Biochemistry, Medicinal, Industrial, Hydrochemistry, Electrochemistry, Green Chemistry, etc. Matter Definition: Anything that occupies space and has definite mass. Physical States: Solid, Liquid, Gaseous, Plasma, Bose-Einstein condensate, Fermionic condensate, Quark-Gluon Plasma. Properties of States: Solids: Orderly arranged particles, definite shape and volume, particles cannot move freely. Liquids: Particles close but can move, definite volume but no definite shape. Gases: Particles far apart, no definite shape or volume, take container's shape and volume. States are interconvertible by changing temperature and pressure. Classification of Matter Matter is classified into Pure Substances and Mixtures based on chemical composition. Pure Substances Contain only one type of particles (e.g., Na, H$_2$O). Classified into Elements and Compounds . Elements: Contain only one type of atom (e.g., H, O). Can be monoatomic (Na, He), diatomic (H$_2$, O$_2$), or polyatomic (P$_4$, S$_8$). Compounds: Contain more than one type of atom combined in a definite ratio (e.g., CO$_2$, H$_2$O). Constituents cannot be separated by physical methods; only by chemical methods. Mixtures Contain more than one type of particles. Components can be separated by physical methods (filtration, crystallization, distillation). Homogeneous Mixtures: Uniform composition throughout (e.g., solutions, air). Components are completely mixed. Heterogeneous Mixtures: Different compositions at different parts (e.g., sea water, soil, muddy water). Significant Figures Meaningful digits known with certainty, plus one uncertain digit. Rules: Non-zero digits are significant (e.g., 285 cm has 3 sig figs). Zeros preceding the first non-zero digit are not significant (e.g., 0.03 has 1 sig fig). Zeros between two non-zero digits are significant (e.g., 2.005 has 4 sig figs). Zeros at the end or right of a number are significant if they are on the right side of the decimal point (e.g., 0.200 g has 3 sig figs). Exact numbers have infinite significant figures (e.g., 2 balls = 2.000000...). In scientific notation, all digits are significant (e.g., $4.01 \times 10^2$ has 3 sig figs). Laws of Chemical Combinations Law of Conservation of Mass (Lavoisier): Matter can neither be created nor destroyed. Total mass of reactants = Total mass of products. Example: $2\text{H}_2(\text{g}) + \text{O}_2(\text{g}) \rightarrow 2\text{H}_2\text{O}(\text{l})$ $(2 \times 2\text{g}) \text{ H}_2 + 32\text{g O}_2 = 2 \times 18\text{g H}_2\text{O}$ $4\text{g} + 32\text{g} = 36\text{g}$ Law of Definite/Constant Proportion (Joseph Proust): A pure compound always contains the same proportion of elements by weight. Example: $\text{CO}_2$ always contains Carbon and Oxygen in a 3:8 ratio by mass, regardless of source. Law of Multiple Proportions (Dalton): When two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other bear a simple whole number ratio. Example: Hydrogen + Oxygen $\rightarrow$ Water ($\text{H}_2\text{O}$) and Hydrogen Peroxide ($\text{H}_2\text{O}_2$). Fixed H mass (2g): O in $\text{H}_2\text{O}$ = 16g, O in $\text{H}_2\text{O}_2$ = 32g. Ratio of oxygen masses: 16:32 or 1:2. Gay-Lussac's Law of Gaseous Volumes: At the same temperature and pressure, when gases combine, their volumes bear a simple whole number ratio. Example: $2\text{H}_2(\text{g}) + \text{O}_2(\text{g}) \rightarrow 2\text{H}_2\text{O}(\text{g})$ Volumes of $\text{H}_2$ and $\text{O}_2$ are in ratio 2:1. Avogadro's Law: Equal volumes of all gases at the same temperature and pressure contain equal numbers of molecules. Dalton's Atomic Theory Matter consists of indivisible atoms. Atoms of the same element are identical; atoms of different elements differ in mass. Atoms can neither be created nor destroyed. Atoms of different elements combine in fixed ratios to form compounds. Atomic Mass and Molecular Mass Atomic Mass: Ratio of mass of an atom to $1/12$th mass of a $\text{C}^{12}$ atom. Atomic mass $= \frac{\text{Mass of one atom of an element}}{1/12 \times \text{Mass of a C}^{12} \text{ atom}}$ Unit: atomic mass unit (amu) or unified mass (u). $1 \text{ amu} = 1.66056 \times 10^{-24} \text{ g}$. Gram Atomic Mass: Atomic mass expressed in grams. Molecular Mass: Sum of atomic masses of elements present in a molecule. Gram Molecular Mass: Molecular mass expressed in grams. For ionic compounds, Formula Mass is used instead of molecular mass. Mole Concept Definition: Amount of substance containing as many particles as there are atoms in 12g of $\text{C}^{12}$. Also defined as the amount of substance containing Avogadro's number of particles. Avogadro's Number: $6.022 \times 10^{23}$ particles/mol. Example: 1 mole of H-atoms = $6.022 \times 10^{23}$ H-atoms. Molar Mass: Mass of one mole of a substance in grams. Percentage Composition Mass % of an element $= \frac{\text{Mass of that element in the compound}}{\text{Molecular mass of the compound}} \times 100$ Empirical and Molecular Formula Empirical Formula: Gives the simplest whole number ratio of atoms in a molecule. Example: E.F. of benzene ($\text{C}_6\text{H}_6$) is CH. Molecular Formula: Gives the actual number of atoms in a molecule. Example: M.F. of benzene = $\text{C}_6\text{H}_6$. Reactions in Solutions (Concentration Terms) Solution: Homogeneous mixture of 2 or more components. Solvent: Component present in larger quantity (or dissolves the solute). Solute: Component present in smaller quantity (or is dissolved). Binary Solution: Solution with only two components. Aqueous Solution: Solution where water is the solvent. Concentration: Amount of solute present in a given volume of solution. Mass Percent Mass percent $= \frac{\text{Mass of solute}}{\text{Mass of solution}} \times 100$ Mole Fraction ($X$) Ratio of moles of a component to the total moles of all components in the solution. For components 1 and 2: $X_1 = \frac{n_1}{n_1 + n_2}$ $X_2 = \frac{n_2}{n_1 + n_2}$ Sum of mole fractions: $X_1 + X_2 = 1$ Molarity ($M$) Number of moles of solute in one liter of solution. $M = \frac{\text{Moles of solute} (n)}{\text{Volume of solution in liters} (V)}$ $M = \frac{w \times 1000}{\text{M.M} \times V_{\text{mL}}}$ (where $w$ = mass of solute, M.M = molecular mass of solute) Unit: mole L$^{-1}$ or Molar (M). Molality ($m$) Number of moles of solute in one kg of solvent. $m = \frac{\text{Moles of solute} (n)}{\text{Mass of solvent in kg} (W)}$ $m = \frac{w \times 1000}{\text{M.M} \times W_{\text{grams}}}$ (where $w$ = mass of solute, M.M = molecular mass of solute) Unit: mole kg$^{-1}$ or molal (m). Limiting Reagent The reagent that gets consumed first and limits the amount of product formed in a chemical reaction. Example: In $2\text{SO}_2(\text{g}) + \text{O}_2(\text{g}) \rightarrow 2\text{SO}_3(\text{g})$, if 10 moles of $\text{SO}_2$ and 10 moles of $\text{O}_2$ are present, $\text{SO}_2$ is the limiting reagent because 10 moles of $\text{SO}_2$ only require 5 moles of $\text{O}_2$ for complete reaction.