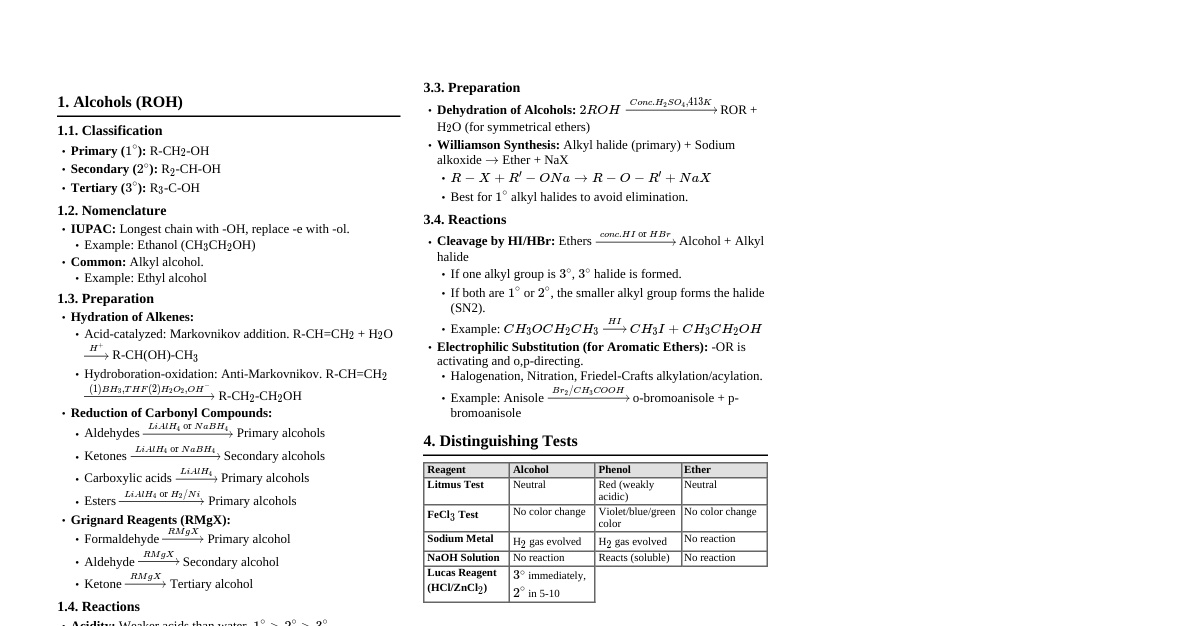
Haloalkanes and Haloarenes Replacement of hydrogen atom(s) in aliphatic/aromatic hydrocarbon by halogen atom(s). Used as solvents for non-polar compounds and starting materials for organic synthesis. 6.1 Classification On the Basis of Number of Halogen Atoms Monohalocompounds: Contain one halogen atom. Dihalocompounds: Contain two halogen atoms. Polyhalocompounds: Contain three or more halogen atoms (tri-, tetra- etc.). Compounds Containing $sp^3$ C-X Bond (a) Alkyl halides or haloalkanes (R—X): Halogen atom bonded to $sp^3$-hybridized carbon atom of an alkyl group. Primary (1°): Halogen attached to primary carbon. R-CH$_2$-X Secondary (2°): Halogen attached to secondary carbon. R-CH(R')-X Tertiary (3°): Halogen attached to tertiary carbon. R-C(R')(R'')-X (b) Allylic halides: Halogen atom bonded to an $sp^3$-hybridized carbon atom adjacent to a C=C double bond (allylic carbon). Example: $CH_2=CH-CH_2-X$ (c) Benzylic halides: Halogen atom bonded to an $sp^3$-hybridized carbon atom attached to an aromatic ring. Example: $C_6H_5-CH_2-X$ Compounds Containing $sp^2$ C-X Bond (a) Vinylic halides: Halogen atom bonded to an $sp^2$-hybridized carbon atom of a C=C double bond. Example: $CH_2=CH-X$ (b) Aryl halides: Halogen atom directly bonded to an $sp^2$-hybridized carbon atom of an aromatic ring. Example: $C_6H_5-X$ 6.2 Nomenclature Common Names: Alkyl group name followed by halide name (e.g., Ethyl chloride). IUPAC Names: Halosubstituted hydrocarbons (e.g., Chloroethane). Dihalogen Derivatives: Ortho (o-), meta (m-), para (p-): Used in common system for substituted benzene derivatives. Numerals (1,2; 1,3; 1,4): Used in IUPAC system. Geminal dihalides (gem-dihalides): Both halogen atoms on the same carbon (e.g., Ethylidene chloride, 1,1-Dichloroethane). Vicinal dihalides (vic-dihalides): Halogen atoms on adjacent carbon atoms (e.g., Ethylene dichloride, 1,2-Dichloroethane). 6.3 Nature of C-X Bond Halogen atoms are more electronegative than carbon, polarizing the C-X bond. Carbon atom bears a partial positive charge ($\delta^+$), halogen atom bears a partial negative charge ($\delta^-$). Bond length increases down the group: C-F Bond enthalpy decreases down the group: C-F > C-Cl > C-Br > C-I. 6.4 Methods of Preparation of Haloalkanes 6.4.1 From Alcohols Reaction with HX: $R-OH + HCl \xrightarrow{ZnCl_2} R-Cl + H_2O$ $R-OH + HBr \longrightarrow R-Br + H_2O$ $R-OH + HI \longrightarrow R-I + H_2O$ Reactivity of alcohols: $3^\circ > 2^\circ > 1^\circ$. Reactivity of hydrogen halides: $HI > HBr > HCl$. Reaction with Phosphorus Halides: $3R-OH + PX_3 \longrightarrow 3R-X + H_3PO_3$ ($X = Cl, Br$) $R-OH + PCl_5 \longrightarrow R-Cl + POCl_3 + HCl$ Reaction with Thionyl Chloride: (Darzen's process) $R-OH + SOCl_2 \longrightarrow R-Cl + SO_2 \uparrow + HCl \uparrow$ Preferred method for alkyl chlorides due to gaseous byproducts. 6.4.2 From Hydrocarbons (I) From Alkanes by Free Radical Halogenation: $CH_3CH_2CH_2CH_3 + Cl_2 \xrightarrow{UV \ light \ or \ heat} CH_3CH_2CH_2CH_2Cl + CH_3CH_2CHClCH_3$ Gives a mixture of isomeric mono- and polyhaloalkanes, difficult to separate. (II) From Alkenes: (i) Addition of Hydrogen Halides: $C=C + HX \longrightarrow H-C-C-X$ $CH_3CH=CH_2 + HI \longrightarrow CH_3CH_2CH_2I$ (minor) $+ CH_3CHICH_3$ (major) Follows Markovnikov's rule. (ii) Addition of Halogens: $CH_2=CH_2 + Br_2 \xrightarrow{CCl_4} BrCH_2-CH_2Br$ Used for detection of double bonds (reddish brown color of $Br_2$ disappears). 6.4.3 Halogen Exchange Finkelstein Reaction: Preparation of alkyl iodides. $R-X + NaI \xrightarrow{dry \ acetone} R-I + NaX \downarrow$ ($X = Cl, Br$) $NaCl$ or $NaBr$ precipitates, shifting equilibrium forward (Le Chatelier's Principle). Swarts Reaction: Preparation of alkyl fluorides. $CH_3Br + AgF \longrightarrow CH_3F + AgBr$ Other metallic fluorides: $Hg_2F_2, CoF_2, SbF_3$. 6.5 Preparation of Haloarenes (i) From Hydrocarbons by Electrophilic Substitution: $C_6H_6 + X_2 \xrightarrow{Fe \ or \ FeX_3} C_6H_5-X + HX$ ($X = Cl, Br$) For substituted benzenes, ortho- and para-isomers are formed. Iodination is reversible and requires an oxidizing agent ($HNO_3, HIO_4$). Fluorocompounds are not prepared by this method due to high reactivity of fluorine. (ii) From Amines by Sandmeyer's Reaction: $Ar-NH_2 \xrightarrow{NaNO_2/HX, 273-278K} Ar-N_2^+X^- \xrightarrow{Cu_2X_2} Ar-X + N_2$ ($X = Cl, Br$) For iodoarenes, $Ar-N_2^+X^- \xrightarrow{KI} Ar-I + N_2$. Physical Properties Boiling Points: Higher than parent hydrocarbons due to greater polarity and stronger intermolecular forces (dipole-dipole and van der Waals). For a given alkyl group: $RI > RBr > RCl > RF$. For isomeric haloalkanes, boiling points decrease with branching. For isomeric dihalobenzenes, para-isomers have higher melting points than ortho- and meta-isomers due to symmetry. Density: Bromo, iodo, and polychloro derivatives are heavier than water. Density increases with number of carbon atoms, halogen atoms, and atomic mass of halogen atoms. Solubility: Slightly soluble in water (less energy released from new attractions than energy required to break existing bonds). Soluble in organic solvents. 6.7 Chemical Reactions 6.7.1 Reactions of Haloalkanes (1) Nucleophilic Substitution Reactions ($S_N$): Nucleophile (electron-rich species) attacks the electron-deficient carbon atom bonded to halogen. Halogen atom acts as a leaving group (halide ion). $S_N2$ (bimolecular nucleophilic substitution): Concerted, one-step reaction. No intermediate formed. Rate depends on concentration of both haloalkane and nucleophile. Inversion of configuration (Walden inversion). Steric hindrance hinders reaction: Methyl halides > Primary halides > Secondary halides > Tertiary halides. $S_N1$ (unimolecular nucleophilic substitution): Two-step reaction. Carbocation intermediate formed in the slow step. Rate depends only on concentration of haloalkane. Racemization occurs due to planar carbocation (attack from either side). Stability of carbocation determines reactivity: Tertiary halides > Secondary halides > Primary halides. Allylic and benzylic halides show high reactivity due to resonance stabilization of carbocations. Ambident Nucleophiles: Possess two nucleophilic centers (e.g., $CN^-, NO_2^-$). $KCN$ (ionic) forms alkyl cyanides (C-C bond). $AgCN$ (covalent) forms alkyl isocyanides (C-N bond). $KNO_2$ forms alkyl nitrites (O-N bond). $AgNO_2$ forms nitroalkanes (N-O bond). (2) Elimination Reactions ($\beta$-Elimination / Dehydrohalogenation): When a haloalkane with $\beta$-hydrogen is heated with alcoholic $KOH$. Hydrogen atom from $\beta$-carbon and halogen from $\alpha$-carbon are removed, forming an alkene. Saytzeff's Rule: The major product is the alkene with the greater number of alkyl groups attached to the doubly bonded carbon atoms. Example: 2-Bromopentane $\xrightarrow{alc.KOH}$ Pent-2-ene (major) + Pent-1-ene (minor) Elimination vs. Substitution: Depends on nature of alkyl halide, strength/size of base/nucleophile, and reaction conditions. Bulky nucleophiles prefer elimination. Primary alkyl halides prefer $S_N2$. Tertiary alkyl halides prefer $S_N1$ or elimination. (3) Reaction with Metals: Grignard Reagents (Alkyl magnesium halides, $RMgX$): $R-X + Mg \xrightarrow{dry \ ether} R-MgX$ Highly reactive, react with any source of proton (e.g., water, alcohols) to form hydrocarbons ($R-MgX + H_2O \longrightarrow R-H + Mg(OH)X$). Must be prepared in dry ether. Wurtz Reaction: $2R-X + 2Na \xrightarrow{dry \ ether} R-R + 2NaX$ Forms hydrocarbons with double the number of carbon atoms. 6.7.2 Reactions of Haloarenes (1) Nucleophilic Substitution Reactions: Extremely less reactive than haloalkanes due to: Resonance effect: C-X bond acquires partial double bond character, making cleavage difficult. Difference in hybridization: Halogen attached to $sp^2$ carbon (more electronegative, shorter, stronger bond) in haloarenes vs. $sp^3$ carbon in haloalkanes. Instability of phenyl cation: Phenyl cation (from $S_N1$) is not resonance stabilized. Possible repulsion: Electron-rich nucleophile repelled by electron-rich arene. Replacement by hydroxyl group: $C_6H_5Cl \xrightarrow{(i) NaOH, 623K, 300 \ atm; (ii) H^+} C_6H_5OH$ Electron withdrawing groups (-$NO_2$) at ortho- and para-positions increase reactivity. (2) Electrophilic Substitution Reactions: Halogen atom is slightly deactivating but ortho-, para-directing due to resonance. (i) Halogenation: $C_6H_5Cl + Cl_2 \xrightarrow{Anhyd. \ FeCl_3} o-Cl_2C_6H_4 + p-Cl_2C_6H_4$ (ii) Nitration: $C_6H_5Cl + HNO_3 \xrightarrow{conc. \ H_2SO_4} o-ClC_6H_4NO_2 + p-ClC_6H_4NO_2$ (iii) Sulphonation: $C_6H_5Cl + conc. \ H_2SO_4 \longrightarrow o-ClC_6H_4SO_3H + p-ClC_6H_4SO_3H$ (iv) Friedel-Crafts Reaction: Alkylation: $C_6H_5Cl + CH_3Cl \xrightarrow{Anhyd. \ AlCl_3} o-ClC_6H_4CH_3 + p-ClC_6H_4CH_3$ Acylation: $C_6H_5Cl + CH_3COCl \xrightarrow{Anhyd. \ AlCl_3} o-ClC_6H_4COCH_3 + p-ClC_6H_4COCH_3$ (3) Reaction with Metals: Wurtz-Fittig Reaction: $Ar-X + R-X + 2Na \xrightarrow{dry \ ether} Ar-R + 2NaX$ (Forms alkylarenes) Fittig Reaction: $2Ar-X + 2Na \xrightarrow{dry \ ether} Ar-Ar + 2NaX$ (Forms diphenyls) 6.8 Polyhalogen Compounds 6.8.1 Dichloromethane (Methylene chloride, $CH_2Cl_2$): Solvent, paint remover, propellant. Harmful to human central nervous system. 6.8.2 Trichloromethane (Chloroform, $CHCl_3$): Solvent for fats, alkaloids. Used to produce refrigerant R-22. Once used as anesthetic. Slowly oxidized by air in presence of light to poisonous phosgene ($COCl_2$). Stored in dark colored bottles, completely filled. 6.8.3 Triiodomethane (Iodoform, $CHI_3$): Antiseptic properties due to liberation of free iodine. Replaced due to objectionable smell. 6.8.4 Tetrachloromethane (Carbon tetrachloride, $CCl_4$): Manufacture of refrigerants, propellants. Solvent, degreasing agent, fire extinguisher. Causes liver cancer, dizziness, nerve damage. Depletes ozone layer. 6.8.5 Freons (Chlorofluorocarbons, CFCs): Extremely stable, unreactive, non-toxic, non-corrosive. Used as aerosol propellants, refrigerants, air conditioning. Deplete ozone layer in stratosphere. 6.8.6 p,p'-Dichlorodiphenyltrichloroethane (DDT): First chlorinated organic insecticide. Effective against mosquitoes (malaria) and lice (typhus). Problems: insect resistance, high toxicity to fish, chemical stability, fat solubility, bioaccumulation. Banned in many countries.