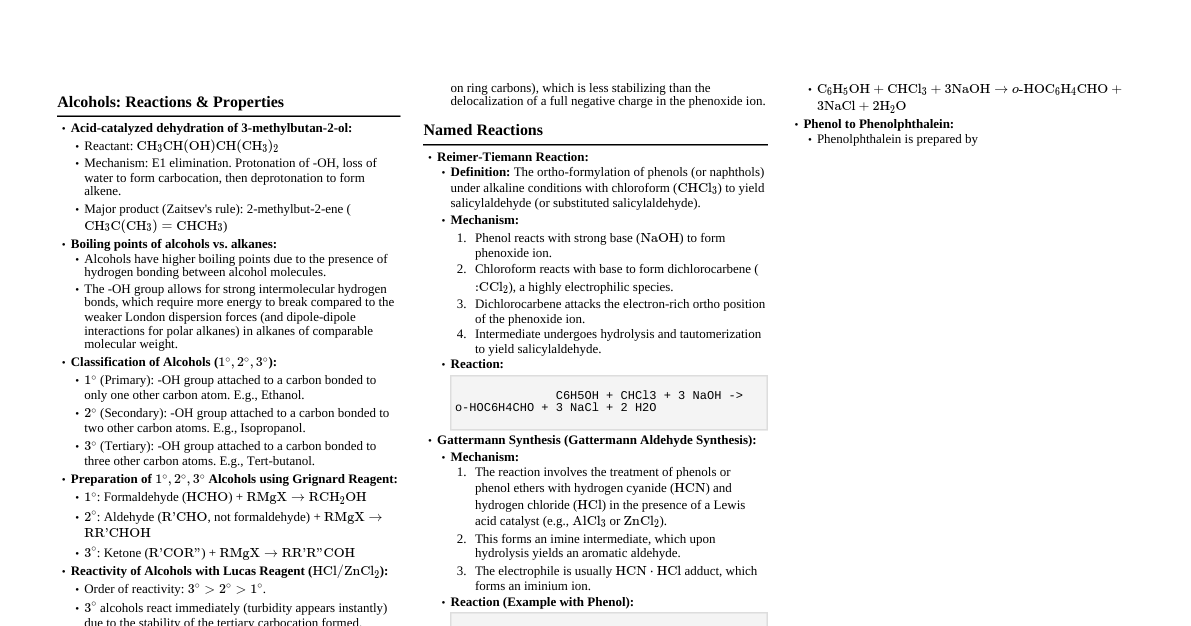
1. Haloalkanes and Haloarenes Nucleophilic Substitution Reactions ($S_N1$ & $S_N2$) $S_N2$: One step, concerted, inversion of configuration. Order: $CH_3X > 1^\circ > 2^\circ$. Substrate: $RX$. Reagent: Nucleophile ($Nu^-$). Example: $CH_3-Br + KOH(aq) \rightarrow CH_3-OH + KBr$ $S_N1$: Two steps, carbocation intermediate, racemization. Order: $3^\circ > 2^\circ > 1^\circ$. Substrate: $RX$. Reagent: Nucleophile ($Nu^-$). Example: $(CH_3)_3C-Br + H_2O \rightarrow (CH_3)_3C-OH + HBr$ Reactions with various Nucleophiles: $KCN \rightarrow R-CN$ (Alkyl Cyanides) $AgCN \rightarrow R-NC$ (Alkyl Isocyanides) $KNO_2 \rightarrow R-ONO$ (Alkyl Nitrites) $AgNO_2 \rightarrow R-NO_2$ (Nitroalkanes) $NaOR' \rightarrow R-O-R'$ (Williamson Synthesis - Ethers) $NH_3 \rightarrow R-NH_2$ (Amines) Elimination Reactions (Dehydrohalogenation) $R-CH_2-CH_2-X \xrightarrow{Alc. KOH, \Delta} R-CH=CH_2 + HX$ Saytzeff's Rule: Major product is the more substituted alkene. Reactions with Metals Wurtz Reaction: $2RX + 2Na \xrightarrow{Dry\ Ether} R-R + 2NaX$ (for alkanes) Wurtz-Fittig Reaction: $Ar-X + RX + 2Na \xrightarrow{Dry\ Ether} Ar-R + 2NaX$ Fittig Reaction: $2Ar-X + 2Na \xrightarrow{Dry\ Ether} Ar-Ar + 2NaX$ Grignard Reagent: $R-X + Mg \xrightarrow{Dry\ Ether} R-Mg-X$ (highly reactive, reacts with compounds having active H) Reactions of Haloarenes Nucleophilic Substitution: Generally difficult due to resonance stabilization and $sp^2$ hybridized carbon. Activated by electron-withdrawing groups (e.g., $-NO_2$) at ortho/para positions. Dow's Process: Chlorobenzene $\xrightarrow{NaOH, 623K, 300atm}$ Phenol Electrophilic Substitution: Halogen is deactivating but o,p-directing. Halogenation: $Ar-X \xrightarrow{X_2, FeCl_3}$ o/p-dihaloarene Nitration: $Ar-X \xrightarrow{Conc. HNO_3/H_2SO_4}$ o/p-nitrohaloarene Sulfonation: $Ar-X \xrightarrow{Conc. H_2SO_4}$ o/p-halobenzenesulfonic acid Friedel-Crafts Alkylation: $Ar-X \xrightarrow{R-Cl, Anhydrous\ AlCl_3}$ o/p-alkylhaloarene Friedel-Crafts Acylation: $Ar-X \xrightarrow{RCOCl, Anhydrous\ AlCl_3}$ o/p-acylhaloarene 2. Alcohols, Phenols, and Ethers Alcohols Acidity: Alcohols are weaker acids than water. Order: $1^\circ > 2^\circ > 3^\circ$ (due to +I effect of alkyl groups). $2R-OH + 2Na \rightarrow 2R-O^-Na^+ + H_2$ Reactions involving C-O bond cleavage: (Reactivity: $3^\circ > 2^\circ > 1^\circ$) With HX (Lucas Test): $R-OH + HX \xrightarrow{ZnCl_2} R-X + H_2O$ With PCl$_3$/PCl$_5$/SOCl$_2$: $R-OH \rightarrow R-Cl$ Dehydration: $R-CH_2-CH_2-OH \xrightarrow{Conc. H_2SO_4, \Delta} R-CH=CH_2 + H_2O$ Reactions involving O-H bond cleavage: (Acidity: $1^\circ > 2^\circ > 3^\circ$) Esterification: $R-OH + R'-COOH \xrightarrow{H^+} R-COOR' + H_2O$ Oxidation: $1^\circ$ Alcohol $\xrightarrow{PCC}$ Aldehyde $\xrightarrow{Strong\ Oxidizing\ Agent}$ Carboxylic Acid $2^\circ$ Alcohol $\xrightarrow{CrO_3\ or\ PCC}$ Ketone $3^\circ$ Alcohols are resistant to oxidation under mild conditions. Catalytic Dehydrogenation: $1^\circ$ Alcohol $\xrightarrow{Cu, 573K}$ Aldehyde $2^\circ$ Alcohol $\xrightarrow{Cu, 573K}$ Ketone $3^\circ$ Alcohol $\xrightarrow{Cu, 573K}$ Alkene (Dehydration) Phenols Acidity: Phenols are more acidic than alcohols (resonance stabilization of phenoxide ion). Reacts with $NaOH$ but not with $NaHCO_3$. Effect of substituents: EWG (like $-NO_2$) increases acidity, EDG (like $-CH_3$) decreases acidity. Electrophilic Substitution Reactions: -OH group is activating and o,p-directing. Nitration: Phenol $\xrightarrow{Dil. HNO_3}$ o/p-nitrophenol; Phenol $\xrightarrow{Conc. HNO_3}$ Picric Acid (2,4,6-trinitrophenol) Bromination: Phenol $\xrightarrow{Br_2/CS_2}$ o/p-bromophenol; Phenol $\xrightarrow{Br_2/H_2O}$ 2,4,6-tribromophenol (white ppt) Kolbe's Reaction: Phenol $\xrightarrow{1.\ NaOH, 2.\ CO_2, 3.\ H^+}$ Salicylic Acid Reimer-Tiemann Reaction: Phenol $\xrightarrow{CHCl_3, NaOH(aq), H^+}$ Salicylaldehyde Friedel-Crafts Reaction: (Alkylation/Acylation) - Difficult due to complexation with catalyst. Oxidation: Phenol $\xrightarrow{Na_2Cr_2O_7/H_2SO_4}$ Benzoquinone Reaction with Zinc Dust: Phenol $\xrightarrow{Zn\ dust, \Delta}$ Benzene Ethers Cleavage of C-O bond: Ethers are relatively unreactive. $R-O-R' + HX \rightarrow R-X + R'-OH$ (Order of reactivity: $HI > HBr > HCl$) If one group is $3^\circ$, $S_N1$ mechanism dominates, $3^\circ$ alkyl halide formed. If both are $1^\circ$ or $2^\circ$, $S_N2$ mechanism, smaller alkyl group forms halide. Electrophilic Substitution (for aromatic ethers, e.g., Anisole): -OR group is activating and o,p-directing. Halogenation: Anisole $\xrightarrow{Br_2/CH_3COOH}$ o/p-bromoanisole Nitration: Anisole $\xrightarrow{Conc. HNO_3/H_2SO_4}$ o/p-nitroanisole Friedel-Crafts Alkylation/Acylation: Anisole $\xrightarrow{R-Cl/RCOCl, Anhydrous\ AlCl_3}$ o/p-alkyl/acyl anisole 3. Aldehydes, Ketones, and Carboxylic Acids Aldehydes and Ketones Nucleophilic Addition Reactions: (Aldehydes are more reactive than ketones due to steric hindrance and electronic effects) Addition of HCN: $\xrightarrow{HCN}$ Cyanohydrins Addition of $\text{NaHSO}_3$: $\xrightarrow{NaHSO_3}$ Bisulphite Addition Product Addition of Grignard Reagents: $\xrightarrow{RMgX, H_3O^+}$ Alcohols (Formaldehyde $\rightarrow 1^\circ$, Aldehydes $\rightarrow 2^\circ$, Ketones $\rightarrow 3^\circ$) Addition of Alcohols: Aldehydes $\xrightarrow{R'OH, H^+ \text{(dry)}}$ Hemiacetals $\xrightarrow{R'OH, H^+ \text{(dry)}}$ Acetals Addition of Ammonia Derivatives: $\xrightarrow{H_2N-Z}$ Imine, Oxime, Hydrazone, Semicarbazone etc. (where Z = -R, -OH, $-NH_2$, $-NHCONH_2$) Reduction: To Alcohols: $\xrightarrow{LiAlH_4\ or\ NaBH_4}$ $1^\circ$ Alcohol (from aldehyde), $2^\circ$ Alcohol (from ketone) To Hydrocarbons: Clemmensen Reduction: $R-CHO/R-CO-R' \xrightarrow{Zn-Hg, Conc. HCl}$ $R-CH_3/R-CH_2-R'$ Wolff-Kishner Reduction: $R-CHO/R-CO-R' \xrightarrow{NH_2NH_2, KOH/Ethylene\ Glycol, \Delta}$ $R-CH_3/R-CH_2-R'$ Oxidation: Aldehydes: Readily oxidized to carboxylic acids. Tollens' Reagent: $R-CHO + 2[Ag(NH_3)_2]^+ + 3OH^- \rightarrow R-COO^- + 2Ag \downarrow + 2H_2O + 4NH_3$ (Silver Mirror) Fehling's Solution: $R-CHO + 2Cu^{2+} + 5OH^- \rightarrow R-COO^- + Cu_2O \downarrow + 3H_2O$ (Red-brown ppt) Strong Oxidizing Agents: $\xrightarrow{KMnO_4, K_2Cr_2O_7}$ Carboxylic Acid Ketones: Oxidized under vigorous conditions (strong agents, high temp) to carboxylic acids with C-C bond cleavage. Reactions due to $\alpha$-hydrogen: Aldol Condensation: Aldehydes/Ketones with $\alpha$-H $\xrightarrow{Dil. NaOH}$ $\beta$-hydroxy aldehyde/ketone $\xrightarrow{\Delta}$ $\alpha, \beta$-unsaturated aldehyde/ketone. Cross Aldol Condensation: Between two different aldehydes/ketones (or one with $\alpha$-H and one without). Haloform Reaction (Iodoform Test): Methyl ketones ($CH_3-CO-R$) and $CH_3CH(OH)-R$ compounds $\xrightarrow{X_2/NaOH}$ $CHX_3$ (Haloform) + $R-COONa$. (For Iodoform, $CHI_3$ is yellow ppt). Other Reactions: Cannizzaro Reaction: Aldehydes without $\alpha$-H $\xrightarrow{Conc. KOH}$ Alcohol + Carboxylate salt. Electrophilic Substitution (Aromatic Aldehydes/Ketones): -CHO/-COR groups are deactivating and meta-directing. Carboxylic Acids Acidity: Stronger acids than phenols and alcohols (due to resonance stabilization of carboxylate ion). React with $Na, NaOH, Na_2CO_3, NaHCO_3$. Effect of substituents: EWG (like $-NO_2, -Cl$) increases acidity, EDG (like $-CH_3$) decreases acidity. Reactions involving cleavage of O-H bond: (Acidic nature) $\xrightarrow{Na/NaOH/Na_2CO_3/NaHCO_3}$ Salt + $H_2/H_2O/CO_2$ Reactions involving cleavage of C-OH bond: Esterification: $R-COOH + R'-OH \xrightarrow{H^+} R-COOR' + H_2O$ Formation of Acid Chlorides: $R-COOH \xrightarrow{PCl_5/SOCl_2}$ $R-COCl$ Formation of Acid Anhydrides: $2R-COOH \xrightarrow{P_2O_5, \Delta}$ $(RCO)_2O$ Reduction: $R-COOH \xrightarrow{LiAlH_4, H_3O^+}$ $R-CH_2OH$ (to $1^\circ$ alcohol) Decarboxylation: $R-COONa \xrightarrow{NaOH + CaO, \Delta}$ $R-H + Na_2CO_3$ Hell-Volhard-Zelinsky (HVZ) Reaction: Carboxylic acids with $\alpha$-H $\xrightarrow{X_2/Red\ P, H_2O}$ $\alpha$-halo carboxylic acid. Electrophilic Substitution (Aromatic Carboxylic Acids): -COOH group is deactivating and meta-directing. 4. Amines Basic Nature of Amines Amines are basic due to the lone pair on nitrogen. Order of basicity (aqueous phase): $2^\circ > 1^\circ > 3^\circ > NH_3$ (for methyl amines); $2^\circ > 3^\circ > 1^\circ > NH_3$ (for ethyl amines). Order of basicity (gas phase): $3^\circ > 2^\circ > 1^\circ > NH_3$. Aromatic amines are less basic than aliphatic amines (due to resonance, lone pair is delocalized). Electron-donating groups increase basicity, electron-withdrawing groups decrease basicity. Amines react with acids to form salts: $R-NH_2 + HCl \rightarrow R-NH_3^+Cl^-$ Reactions of Amines Alkylation: $R-NH_2 \xrightarrow{R'X}$ $R-NHR'$ (secondary) $\xrightarrow{R'X}$ $R-NR'_2$ (tertiary) $\xrightarrow{R'X}$ $R-N^+R'_3X^-$ (quaternary ammonium salt). Acylation: $R-NH_2 \xrightarrow{R'COCl\ or\ (R'CO)_2O}$ Amide ($R-NH-COR'$). (Tertiary amines do not undergo acylation). Carbylamine Reaction (Isocyanide Test): $1^\circ$ Amines (aliphatic & aromatic) $\xrightarrow{CHCl_3, Alc. KOH, \Delta}$ Alkyl/Aryl isocyanide (foul smell). Reaction with Nitrous Acid ($HNO_2$): $1^\circ$ Aliphatic Amine $\xrightarrow{NaNO_2/HCl}$ Alcohol + $N_2$ gas. $1^\circ$ Aromatic Amine $\xrightarrow{NaNO_2/HCl, 0-5^\circ C}$ Arenediazonium salt ($Ar-N_2^+Cl^-$). $2^\circ$ Amine $\xrightarrow{HNO_2}$ N-nitrosoamine (yellow oily product). $3^\circ$ Aliphatic Amine $\xrightarrow{HNO_2}$ No visible reaction, forms salt. $3^\circ$ Aromatic Amine $\xrightarrow{HNO_2}$ p-nitroso-N,N-dialkylaniline (green solution). Hinsberg's Test (Distinction of $1^\circ, 2^\circ, 3^\circ$ Amines): Reagent: Benzenesulphonyl chloride ($C_6H_5SO_2Cl$). $1^\circ$ Amine: Forms N-alkylbenzenesulphonamide, soluble in $KOH$. $2^\circ$ Amine: Forms N,N-dialkylbenzenesulphonamide, insoluble in $KOH$. $3^\circ$ Amine: No reaction. Electrophilic Substitution Reactions (Aromatic Amines): $-NH_2$ group is strongly activating and o,p-directing. Bromination: Aniline $\xrightarrow{Br_2/H_2O}$ 2,4,6-tribromoaniline (white ppt). To get mono-substituted product, $-\text{NH}_2$ activated by acetylation (e.g., Aniline $\xrightarrow{Ac_2O}$ Acetanilide $\xrightarrow{Br_2/CH_3COOH}$ p-bromoacetanilide $\xrightarrow{H_2O/H^+}$ p-bromoaniline). Nitration: Direct nitration gives tarry products and p-nitroaniline (due to oxidation and anilinium ion formation). To get mono-substituted product, protect $-\text{NH}_2$ by acetylation. Sulfonation: Aniline $\xrightarrow{Conc. H_2SO_4}$ Anilinium hydrogensulphate $\xrightarrow{\Delta}$ Sulphanilic acid (zwitterionic form). Friedel-Crafts Reaction: Not possible due to salt formation with $AlCl_3$. 5. Biomolecules (Chemical Properties) Carbohydrates Monosaccharides (Glucose, Fructose): Reducing Sugars: Due to free aldehyde/ketone group, reduce Tollens' and Fehling's reagents. Reaction with HCN: Forms cyanohydrin. Reaction with Hydroxylamine: Forms oxime. Oxidation: $\xrightarrow{Br_2/H_2O}$ Gluconic acid (aldehyde group oxidized). $\xrightarrow{HNO_3}$ Saccharic acid (both aldehyde & primary alcohol groups oxidized). Reduction: $\xrightarrow{NaBH_4\ or\ H_2/Ni}$ Glucitol (Sorbitol) (aldehyde group reduced to alcohol). Disaccharides (Sucrose, Maltose, Lactose): Sucrose: Non-reducing sugar (glycosidic linkage involves both anomeric carbons). On hydrolysis ($\xrightarrow{H^+ \text{ or invertase}}$), gives glucose + fructose (invert sugar). Maltose, Lactose: Reducing sugars (free hemiacetal/hemiketal group). Polysaccharides (Starch, Cellulose): Non-reducing. On hydrolysis, yield monosaccharides. Starch: Gives blue color with Iodine solution. Cellulose: Does not give color with Iodine. Proteins Hydrolysis: Proteins $\xrightarrow{H^+ \text{ or enzymes}}$ $\alpha$-amino acids. Denaturation: Loss of biological activity and change in secondary/tertiary structure due to pH change, temperature, etc. (e.g., coagulation of egg white on boiling). Tests for Proteins: Biuret test: Gives violet color with $CuSO_4$ and $NaOH$. Ninhydrin test: Gives purple color with amino acids. Nucleic Acids (DNA, RNA) Hydrolysis: Nucleic acid $\rightarrow$ Pentose sugar + Phosphoric acid + Nitrogenous bases. Denaturation (DNA): Separation of double helix into single strands at high temperature or extreme pH (melting). Renaturation: Reforming of double helix upon cooling. Vitamins Specific deficiency diseases for each vitamin. Water-soluble (B, C) vs. Fat-soluble (A, D, E, K). Hormones Specific physiological functions. Steroids, peptides, catecholamines.