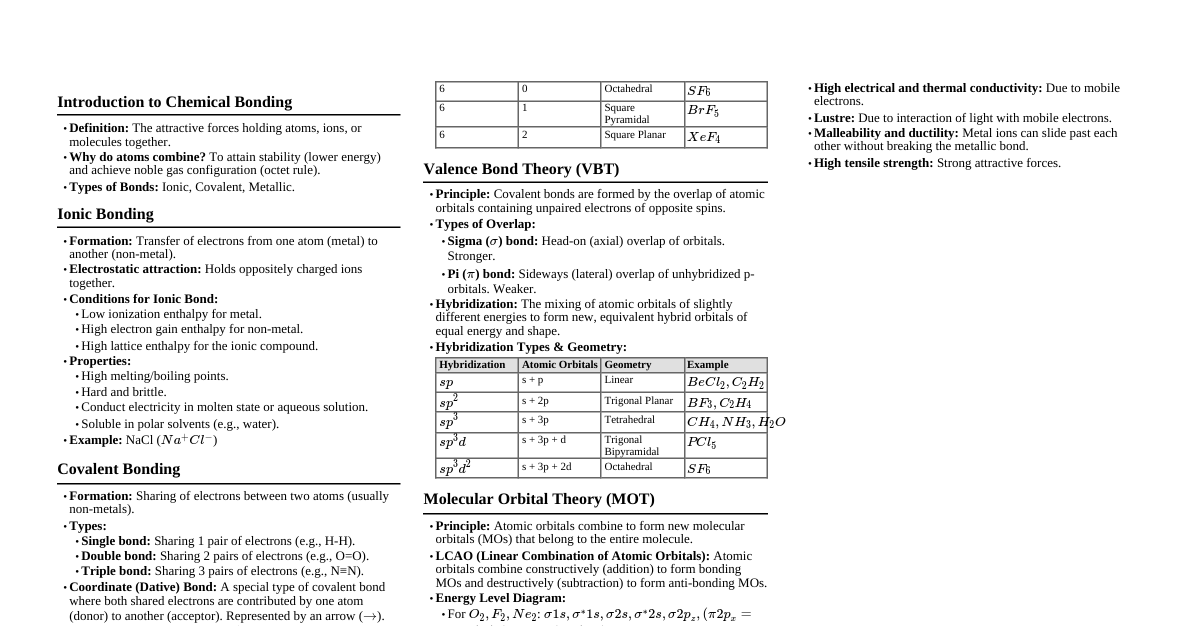
Chemical Bond: Introduction Attractive force binding atoms together. Formed by electron transfer or sharing. Lewis Symbols Represent valence electrons (outermost electrons) as dots around element symbol. Inner electrons are core electrons. Examples: Li: $Li \cdot$ Be: $\cdot Be \cdot$ B: $\cdot \dot{B} \cdot$ C: $\cdot \ddot{C} \cdot$ N: $\cdot \ddot{N} \cdot$ O: $\cdot \ddot{O} \cdot \cdot$ Ne: $: \ddot{Ne} :$ Octet Rule Atoms react to achieve 8 electrons in their valence shell (octet). Atoms with 8 valence electrons are stable. Covalent Bond (Langmuir) Formed by sharing of electrons. Examples: H-H, Cl-Cl, $H_2O$, $CH_4$ Single Bond: One pair of shared electrons. $: \ddot{Cl} \cdot + \cdot \ddot{Cl} : \longrightarrow : \ddot{Cl} : \ddot{Cl} :$ chlorine atoms $\longrightarrow$ chlorine molecule Double Bond: Two pairs of shared electrons. Example: $CO_2$ $: \ddot{O} :: C :: \ddot{O} :$ or $: O = C = O :$ Each atom achieves an octet (8e-). Triple Bond: Three pairs of shared electrons. Example: $N_2$ $: N ::: N :$ Lone Pairs Electron pairs that do not participate in bonding. Examples: N-atom: one lone pair O-atom: two lone pairs Cl-atom: three lone pairs Ionic or Electrovalent Bond Formed by complete transfer of electrons between two atoms. Results in oppositely charged ions attracting each other. Conditions: Low ionization enthalpy for atom losing electron, high electron gain enthalpy for atom gaining electron. Examples: NaCl, $CaF_2$ Na ($1s^2 2s^2 2p^6 3s^1$) $\longrightarrow$ $Na^+$ ($1s^2 2s^2 2p^6$) (octet) Cl ($1s^2 2s^2 2p^6 3s^2 3p^5$) $\longrightarrow$ $Cl^-$ ($1s^2 2s^2 2p^6 3s^2 3p^6$) (octet) $Na^+ + Cl^- \longrightarrow NaCl$ Lattice Enthalpy Energy change when one mole of an ionic solid is formed from its gaseous ions. OR energy required to break one mole of an ionic solid into its gaseous constituent ions. Formal Charge Formal charge = (No. of valence electrons) - (No. of lone pair electrons) - $\frac{1}{2}$ (No. of bonding electrons) Example: Ozone ($O_3$) Limitations of Octet Rule Sub-octet: Central atom has less than 8 electrons. Examples: $BeCl_2$, $BF_3$ Odd electron molecules: Examples: NO, $NO_2$ Super octet (expanded octet): Central atom has more than 8 electrons. Examples: $PCl_5$, $SF_6$ Fails to explain molecule shape, energy, and stability of sub/super octet molecules. Bond Parameters Bond Length: Distance between nuclei of two bonded atoms. Bond Angle: Angle between bonding pairs around the central atom. Bond Enthalpy: Energy required to break one mole of bonds in gaseous state, or energy released when one mole of bond is formed. For polyatomic molecules, average bond enthalpy is used. Bond Order: Number of bonds between two atoms. Examples: $H_2$ (1), $O_2$ (2), $N_2$ (3). Isoelectronic species have same bond order. Resonance When a single Lewis structure cannot represent a molecule, the actual structure is a hybrid of different canonical structures (resonating structures). Canonical structures have similar energy, nuclei positions, and bonding/non-bonding electron pairs. Resonance stabilizes the molecule. Example: Ozone ($O_3$) 148 pm 121 pm 121 pm 148 pm I II III Polarity of Bonds Dipole Moment ($\mu$) Definition: Product of charge (Q) and distance of separation (r) between positive and negative centers. $\mu = Q \times r$ Unit: Debye (D). Possesses both magnitude and direction. For polyatomic molecules, dipole moment depends on bond dipoles and molecular shape. Zero dipole moment for: $CO_2$, $BeCl_2$, $BeF_2$, $BF_3$, $CCl_4$. Example: $H_2O$ has net dipole moment of 1.85 D. $NH_3$ vs $NF_3$: Dipole moment of $NH_3$ (4.90 D) is greater than $NF_3$ (0.80 D). In $NH_3$, lone pair dipole and N-H bond dipoles add up. In $NF_3$, greater electronegativity of F causes orbital dipole to oppose the N-F bond dipoles. Fajans Rule (Covalent Character in Ionic Bonds) Smaller cation size, larger anion size $\longrightarrow$ greater covalent character. Greater cation charge $\longrightarrow$ greater covalent character. For cations of same charge/size, those with $(n-1)d^n ns^0$ configuration have more covalent character than $ns^2 np^6$ configuration. Valence Shell Electron Pair Repulsion Theory (VSEPR) Molecular shape depends on the number of valence shell electron pairs around the central atom. Electron pairs repel each other. Electron pairs occupy positions to minimize repulsions and maximize distance apart. Repulsive forces order: Lone pair-Lone pair (lp-lp) $>$ Lone pair-Bond pair (lp-bp) $>$ Bond pair-Bond pair (bp-bp). Valence Bond Theory (VBT) Bond formation occurs by overlap of atomic orbitals. Formation of $H_2$ molecule: Consider two H-atoms, $H_A$ and $H_B$, with nuclei $N_A, N_B$ and electrons $e_A, e_B$. Attractive forces: $N_A-e_A$, $N_B-e_B$, $N_A-e_B$, $N_B-e_A$. Repulsive forces: $e_A-e_B$, $N_A-N_B$. At a specific internuclear distance, attractive forces maximize, leading to bond formation and energy release (bond enthalpy). Bond length for $H_2$: 74 pm; Bond enthalpy: 435.8 kJ/mol. Greater orbital overlap leads to stronger bonds. Types of Bonds: Sigma ($\sigma$) bond Formed by axial (head-on) overlapping of atomic orbitals. Examples: s-s overlap, s-p overlap, p-p (axial) overlap. Pi ($\pi$) bond Formed by lateral (sideways) overlapping of p-orbitals (perpendicular to internuclear axis). $\sigma$ bond is stronger than $\pi$ bond. Hybridisation Intermixing of atomic orbitals of slightly different energies to form new hybrid orbitals of equivalent energy, size, and shape. Types of Hybridization: sp Hybridization (Diagonal Hybridization) Intermixing of one s and one p orbital to form two equivalent sp hybrid orbitals. Example: $BeCl_2$ Be (ground state): $1s^2 2s^2$ Be (excited state): $1s^2 2s^1 2p^1$ Cl: $1s^2 2s^2 2p^6 3s^2 3p^5$ The two sp hybrid orbitals of Be overlap with 3p orbitals of two Cl atoms to form two Be-Cl $\sigma$ bonds. Shape: Linear, Bond angle: $180^\circ$. Example: $C_2H_2$ (Ethyne/Acetylene) Each C atom undergoes sp hybridization. One C-C $\sigma$ bond, two C-H $\sigma$ bonds. Two unhybridized p orbitals on each C atom form two $\pi$ bonds. Total: 3 $\sigma$ bonds and 2 $\pi$ bonds. Shape: Linear. $sp^2$ Hybridization Intermixing of one s and two p orbitals to form three equivalent $sp^2$ hybrid orbitals. Example: $BCl_3$ B (ground state): $1s^2 2s^2 2p^1$ B (excited state): $1s^2 2s^1 2p^2$ The three $sp^2$ hybrid orbitals of B overlap with 3p orbitals of three Cl atoms to form three B-Cl $\sigma$ bonds. Shape: Trigonal planar, Bond angle: $120^\circ$. Example: $C_2H_4$ (Ethene) Each C atom undergoes $sp^2$ hybridization. One C-C $\sigma$ bond, four C-H $\sigma$ bonds. One unhybridized p orbital on each C atom forms one $\pi$ bond. Total: 5 $\sigma$ bonds and 1 $\pi$ bond. $sp^3$ Hybridization Intermixing of one s and three p orbitals to form four equivalent $sp^3$ hybrid orbitals. Example: $CH_4$ (Methane) C (ground state): $1s^2 2s^2 2p^2$ C (excited state): $1s^2 2s^1 2p^3$ The four $sp^3$ hybrid orbitals of C overlap with 1s orbitals of four H atoms to form four C-H $\sigma$ bonds. Shape: Tetrahedral, Bond angle: $109^\circ 28'$. Example: $C_2H_6$ (Ethane) Each C atom undergoes $sp^3$ hybridization. One C-C $\sigma$ bond, six C-H $\sigma$ bonds. Shape: Tetrahedral around each C. Example: $NH_3$ (Ammonia) N (ground state): $1s^2 2s^2 2p^3$ N undergoes $sp^3$ hybridization. One $sp^3$ orbital contains a lone pair, and three $sp^3$ orbitals form 3 N-H $\sigma$ bonds. Lone pair-bond pair repulsion causes distortion. Shape: Pyramidal, Bond angle: $107^\circ$. Example: $H_2O$ (Water) O (ground state): $1s^2 2s^2 2p^4$ O undergoes $sp^3$ hybridization. Two $sp^3$ orbitals contain lone pairs, and two $sp^3$ orbitals form 2 O-H $\sigma$ bonds. Lone pair-lone pair and lone pair-bond pair repulsions cause distortion. Shape: Bent or 'V' (angular), Bond angle: $104.5^\circ$. $sp^3d$ Hybridization Intermixing of one s, three p, and one d orbital to form five equivalent $sp^3d$ hybrid orbitals. Directed towards corners of a trigonal bipyramid. Bond angles: $90^\circ$ (axial-equatorial), $120^\circ$ (equatorial). Example: $PCl_5$ P (ground state): $3s^2 3p^3$ P (excited state): $3s^1 3p^3 3d^1$ Five $sp^3d$ hybrid orbitals of P overlap with 3p orbitals of five Cl atoms. Equatorial bonds repel axial bonds, making $PCl_5$ reactive ($PCl_5 \longrightarrow PCl_3 + Cl_2$). $sp^3d^2$ Hybridization Intermixing of one s, three p, and two d orbitals to form six equivalent $sp^3d^2$ hybrid orbitals. Directed towards corners of an octahedron. Bond angle: $90^\circ$. Example: $SF_6$ S (ground state): $3s^2 3p^4$ S (excited state): $3s^1 3p^3 3d^2$ Six $sp^3d^2$ hybrid orbitals of S overlap with p orbitals of six F atoms to form 6 S-F bonds. Shape: Octahedral. Molecular Orbital Theory (MOT) - Hund and Mulliken Postulates: Atomic orbitals of comparable energy and proper symmetry combine to form molecular orbitals (MOs). Atomic orbitals are monocentric; MOs are polycentric. Number of MOs formed equals number of combining atomic orbitals. Two types: Bonding Molecular Orbital (BMO) and Antibonding Molecular Orbital (ABMO). BMOs have lower energy and greater stability than ABMOs. MOs describe electron probability around groups of nuclei. MOs are filled according to Aufbau principle, Pauli's exclusion principle, and Hund's rule. Formation of MOs - Linear Combination of Atomic Orbitals (LCAO) Method: Combining two atomic orbitals ($\Psi_A, \Psi_B$) results in two MOs: Bonding MO ($\Psi_{BMO}$): Formed by in-phase combination ($\Psi_A + \Psi_B$). Increased electron density between nuclei, high stability. Antibonding MO ($\Psi_{ABMO}$): Formed by out-of-phase combination ($\Psi_A - \Psi_B$). Decreased electron density between nuclei (node), lower stability. Conditions for combination of atomic orbitals: Similar energy. Same symmetry. Maximum overlap. Energy Levels of Molecular Orbitals: Up to $N_2$: $\sigma 1s From $O_2$ onwards: $\sigma 1s Bond Order (MOT) $B.O. = \frac{1}{2} (N_b - N_a)$ $N_b$: number of electrons in bonding MOs; $N_a$: number of electrons in antibonding MOs. B.O. can be positive, zero, or negative. B.O. = 1, 2, 3 correspond to single, double, and triple bonds, respectively. Higher bond order $\longrightarrow$ greater stability, shorter bond length. Magnetic Properties: Diamagnetic: All molecular orbitals are doubly occupied (no unpaired electrons). Paramagnetic: One or more molecular orbitals are singly occupied (unpaired electrons). Bonding in homonuclear diatomic molecules: $H_2$: $(\sigma 1s)^2$ $B.O. = \frac{1}{2}(2-0) = 1$. Diamagnetic. $He_2$: $(\sigma 1s)^2 (\sigma^*1s)^2$ $B.O. = \frac{1}{2}(2-2) = 0$. Unstable, does not exist. $Li_2$: $(\sigma 1s)^2 (\sigma^*1s)^2 (\sigma 2s)^2$ $B.O. = \frac{1}{2}(4-2) = 1$. $O_2$: $(\sigma 1s)^2 (\sigma^*1s)^2 (\sigma 2s)^2 (\sigma^*2s)^2 (\sigma 2p_z)^2 (\pi 2p_x)^2 (\pi 2p_y)^2 (\pi^*2p_x)^1 (\pi^*2p_y)^1$ $B.O. = \frac{1}{2}(10-6) = 2$. Paramagnetic (due to two unpaired electrons). Hydrogen Bonding Attractive force between a hydrogen atom of one molecule and an electronegative atom (F, O, or N) of the same or another molecule. Types of H-bonds: Intermolecular Hydrogen Bond Between two molecules of the same or different compounds. Examples: HF, $H_2O$, $NH_3$. $\delta^+ H - \delta^- F \cdots \delta^+ H - \delta^- F \cdots \delta^+ H - \delta^- F$ Intramolecular Hydrogen Bond Within the same molecule. Example: o-nitrophenol.