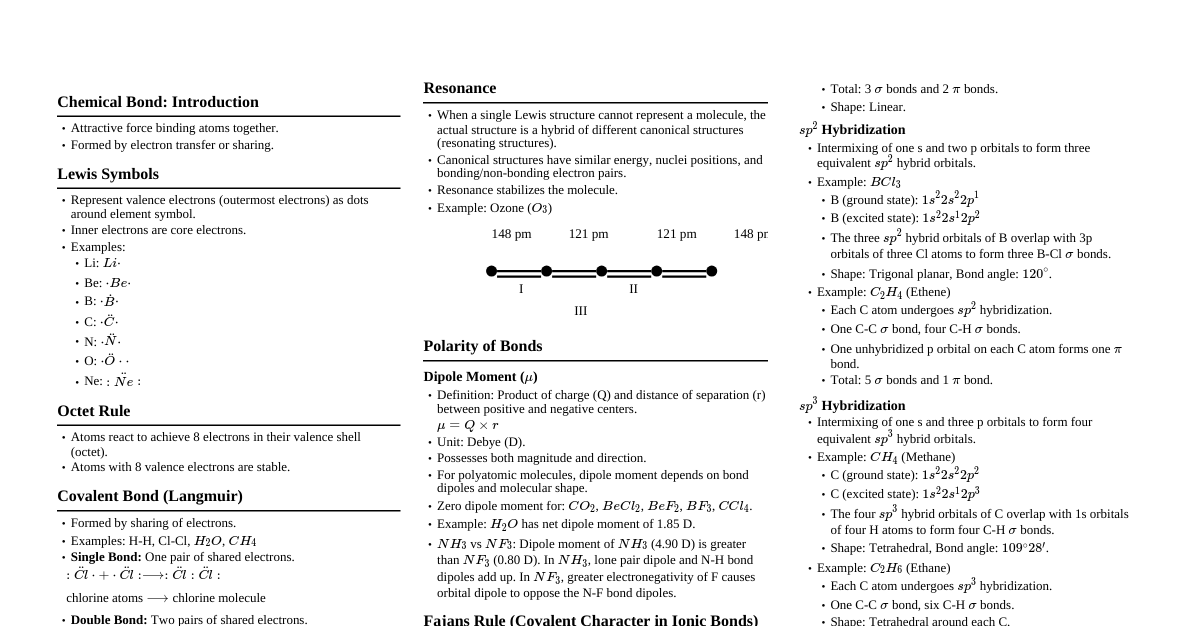
1. Lewis Dot Structures Represent valence electrons as dots around the atomic symbol. Octet Rule: Atoms tend to gain, lose, or share electrons to achieve 8 valence electrons (except H, Li, Be). Formal Charge ($FC$): $FC = (\text{valence electrons}) - (\text{non-bonding electrons}) - \frac{1}{2}(\text{bonding electrons})$ Minimize formal charges for the most stable structure. 2. Ionic Bonding Transfer of electrons from metal to non-metal. Large electronegativity difference ($\Delta EN > 1.7$). Forms cations and anions, held by electrostatic forces. Lattice Energy: Energy released when gaseous ions combine to form one mole of solid ionic compound. Factors: Charge of ions (higher $\propto$ Lattice Energy), size of ions (smaller $\propto$ Lattice Energy). 3. Covalent Bonding Sharing of electrons between two non-metals. Small or no electronegativity difference ($\Delta EN Types: Non-polar Covalent: Equal sharing of electrons ($\Delta EN \approx 0$). E.g., $\text{H}_2$, $\text{Cl}_2$. Polar Covalent: Unequal sharing of electrons ($0 Bond Length: Average distance between nuclei of two bonded atoms. Factors: Bond order (higher $\propto$ shorter), atomic size (smaller $\propto$ shorter). Bond Energy: Energy required to break one mole of a particular bond. Factors: Bond order (higher $\propto$ stronger), atomic size (smaller $\propto$ stronger). 4. Dipole Moment ($\mu$) Measure of polarity of a molecule. $\mu = q \times d$ (charge $\times$ distance). Unit: Debye (D). Vector quantity. Net dipole moment determines molecular polarity. Symmetrical molecules with polar bonds can be non-polar overall (e.g., $\text{CO}_2$, $\text{CCl}_4$). Asymmetrical molecules with polar bonds are usually polar (e.g., $\text{H}_2\text{O}$, $\text{NH}_3$). 5. VSEPR Theory (Valence Shell Electron Pair Repulsion) Predicts molecular geometry based on minimizing electron pair repulsion around central atom. Electron pairs (lone pairs + bond pairs) arrange as far apart as possible. Lone pair-lone pair repulsion > Lone pair-bond pair repulsion > Bond pair-bond pair repulsion. Steric No. Bond Pairs Lone Pairs Geometry Example 2 2 0 Linear $\text{BeCl}_2$, $\text{CO}_2$ 3 3 0 Trigonal Planar $\text{BF}_3$, $\text{SO}_3$ 3 2 1 Bent $\text{SO}_2$ 4 4 0 Tetrahedral $\text{CH}_4$, $\text{SiCl}_4$ 4 3 1 Trigonal Pyramidal $\text{NH}_3$ 4 2 2 Bent $\text{H}_2\text{O}$ 5 5 0 Trigonal Bipyramidal $\text{PCl}_5$ 5 4 1 Seesaw $\text{SF}_4$ 5 3 2 T-shaped $\text{ClF}_3$ 5 2 3 Linear $\text{XeF}_2$ 6 6 0 Octahedral $\text{SF}_6$ 6 5 1 Square Pyramidal $\text{BrF}_5$ 6 4 2 Square Planar $\text{XeF}_4$ 6. Hybridization Mixing of atomic orbitals to form new hybrid orbitals with different energies and shapes. Count steric number (SN = number of $\sigma$ bonds + number of lone pairs). SN = 2 $\implies sp$ hybridization (linear) SN = 3 $\implies sp^2$ hybridization (trigonal planar) SN = 4 $\implies sp^3$ hybridization (tetrahedral) SN = 5 $\implies sp^3d$ hybridization (trigonal bipyramidal) SN = 6 $\implies sp^3d^2$ hybridization (octahedral) 7. Valence Bond Theory (VBT) Covalent bonds form by overlap of atomic orbitals. $\sigma$ (sigma) bonds: Head-on overlap, rotations allowed around bond axis. Stronger. $\pi$ (pi) bonds: Sideways overlap of unhybridized p-orbitals. Weaker, restricted rotation. Single bond: $1\sigma$ Double bond: $1\sigma + 1\pi$ Triple bond: $1\sigma + 2\pi$ 8. Molecular Orbital Theory (MOT) Atomic orbitals combine to form molecular orbitals (MOs) that span the entire molecule. Linear Combination of Atomic Orbitals (LCAO) method. Number of MOs formed = Number of atomic orbitals combined. Bonding MOs (lower energy, constructive interference) and Anti-bonding MOs (higher energy, destructive interference). Order of filling MOs (Aufbau, Pauli, Hund's rules apply). For $\text{N}_2$ and lighter (B, C, N): $\sigma 1s, \sigma^* 1s, \sigma 2s, \sigma^* 2s, (\pi 2p_x = \pi 2p_y), \sigma 2p_z, (\pi^* 2p_x = \pi^* 2p_y), \sigma^* 2p_z$ For $\text{O}_2$ and heavier (O, F, Ne): $\sigma 1s, \sigma^* 1s, \sigma 2s, \sigma^* 2s, \sigma 2p_z, (\pi 2p_x = \pi 2p_y), (\pi^* 2p_x = \pi^* 2p_y), \sigma^* 2p_z$ Bond Order ($\text{BO}$): $\text{BO} = \frac{1}{2} (\text{No. of electrons in bonding MOs} - \text{No. of electrons in anti-bonding MOs})$ Magnetic properties: Paramagnetic: Contains unpaired electrons (attracted to magnetic field). Diamagnetic: All electrons are paired (repelled by magnetic field). 9. Hydrogen Bonding Special dipole-dipole interaction between H atom bonded to a highly electronegative atom (F, O, N) and a lone pair on another F, O, N atom. Intramolecular H-bonding: Within the same molecule (e.g., o-nitrophenol). Intermolecular H-bonding: Between different molecules (e.g., $\text{H}_2\text{O}$, $\text{NH}_3$, $\text{HF}$). Increases boiling point, melting point, viscosity, surface tension, and solubility in water. 10. Metallic Bonding Electrostatic attraction between a "sea" of delocalized valence electrons and positively charged metal ions. Accounts for properties like conductivity, malleability, ductility, luster. 11. Important Exceptions & Concepts Incomplete Octet: $\text{BeH}_2$, $\text{BF}_3$, $\text{AlCl}_3$. Expanded Octet: $\text{PCl}_5$, $\text{SF}_6$, $\text{IF}_7$, $\text{XeF}_2$, $\text{XeF}_4$. (Elements from 3rd period onwards). Odd Electron Molecules: $\text{NO}$, $\text{NO}_2$ (paramagnetic). Isoelectronic Species: Same number of electrons, similar bonding/geometry (e.g., $\text{CO}$, $\text{N}_2$; $\text{NO}_3^-$, $\text{CO}_3^{2-}$). Resonance: Delocalization of $\pi$ electrons. Actual structure is a resonance hybrid. Rules: Same atomic positions, same number of lone pairs, same number of unpaired electrons. Contributes to stability.