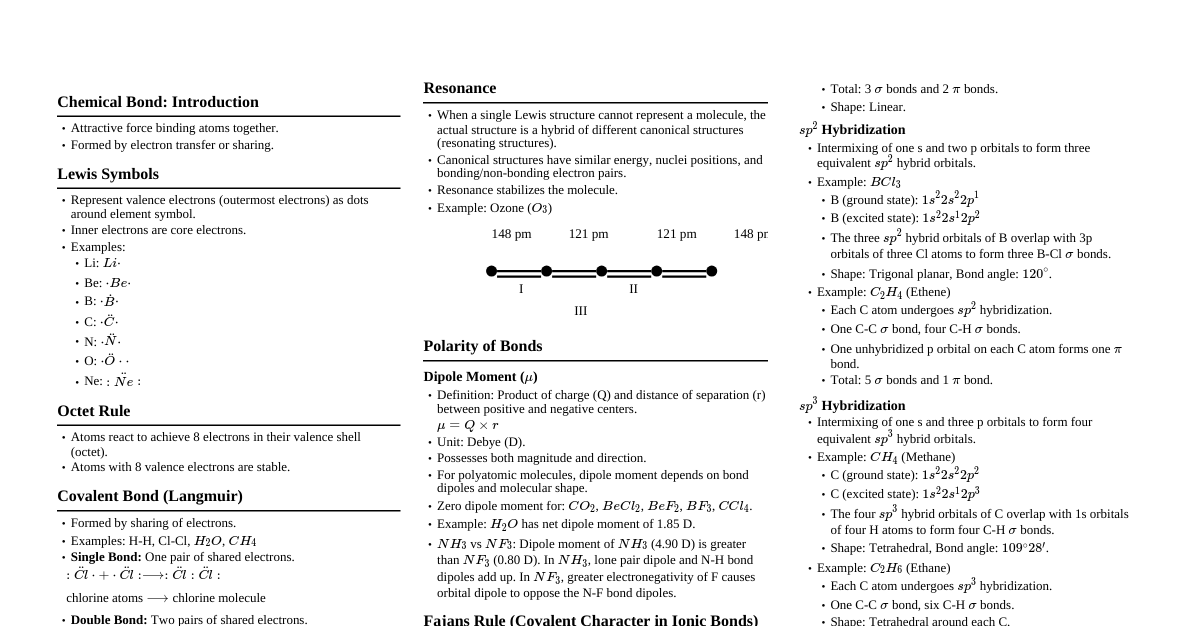
### Introduction to Chemical Bonding - **Definition:** The attractive forces holding atoms, ions, or molecules together. - **Why do atoms combine?** To attain stability (lower energy) and achieve noble gas configuration (octet rule). - **Types of Bonds:** Ionic, Covalent, Metallic. ### Ionic Bonding - **Formation:** Transfer of electrons from one atom (metal) to another (non-metal). - **Electrostatic attraction:** Holds oppositely charged ions together. - **Conditions for Ionic Bond:** - Low ionization enthalpy for metal. - High electron gain enthalpy for non-metal. - High lattice enthalpy for the ionic compound. - **Properties:** - High melting/boiling points. - Hard and brittle. - Conduct electricity in molten state or aqueous solution. - Soluble in polar solvents (e.g., water). - **Example:** NaCl ($Na^+ Cl^-$) ### Covalent Bonding - **Formation:** Sharing of electrons between two atoms (usually non-metals). - **Types:** - **Single bond:** Sharing 1 pair of electrons (e.g., H-H). - **Double bond:** Sharing 2 pairs of electrons (e.g., O=O). - **Triple bond:** Sharing 3 pairs of electrons (e.g., N≡N). - **Coordinate (Dative) Bond:** A special type of covalent bond where both shared electrons are contributed by one atom (donor) to another (acceptor). Represented by an arrow ($\rightarrow$). - **Example:** Formation of $NH_4^+$ from $NH_3$ and $H^+$. - **Properties:** - Generally lower melting/boiling points than ionic compounds. - Poor conductors of electricity. - May be soluble in non-polar solvents. - Can be gases, liquids, or solids. ### Lewis Structures - **Representation:** Shows valence electrons as dots and bonds as lines. - **Steps to draw:** 1. Count total valence electrons. 2. Identify central atom (least electronegative, usually). 3. Draw single bonds to peripheral atoms. 4. Complete octets of peripheral atoms. 5. Place remaining electrons on central atom. 6. If central atom lacks octet, form multiple bonds. - **Formal Charge:** $FC = V - N_L - \frac{1}{2}N_B$ - $V$: Valence electrons - $N_L$: Non-bonding (lone pair) electrons - $N_B$: Bonding electrons - Sum of formal charges must equal charge of ion/molecule. - Prefer structures with formal charges closest to zero. ### Octet Rule & Its Limitations - **Octet Rule:** Atoms tend to gain, lose, or share electrons to achieve 8 electrons in their valence shell. - **Limitations:** - **Incomplete Octet:** Less than 8 valence electrons (e.g., $LiCl, BeH_2, BCl_3$). - **Expanded Octet:** More than 8 valence electrons (e.g., $PCl_5, SF_6, H_2SO_4$). Occurs for elements in Period 3 and beyond due to availability of d-orbitals. - **Odd Electron Molecules:** Molecules with an odd number of valence electrons (e.g., $NO, NO_2$). - **Noble Gases:** Some noble gases form compounds (e.g., $XeF_2, XeF_4$). ### VSEPR Theory - **Principle:** Electron pairs (both bonding and lone pairs) around the central atom repel each other and arrange themselves to minimize repulsion. - **Predicts:** Geometry of molecules. - **Repulsion Order:** Lone Pair-Lone Pair (LP-LP) > Lone Pair-Bond Pair (LP-BP) > Bond Pair-Bond Pair (BP-BP). - **Molecular Geometry Examples:** | Electron Pairs (BP+LP) | Lone Pairs (LP) | Geometry | Example | |------------------------|-----------------|------------------|-----------------| | 2 | 0 | Linear | $BeCl_2, CO_2$ | | 3 | 0 | Trigonal Planar | $BF_3, CO_3^{2-}$ | | 3 | 1 | Bent (V-shaped) | $SO_2$ | | 4 | 0 | Tetrahedral | $CH_4, NH_4^+$ | | 4 | 1 | Trigonal Pyramidal| $NH_3$ | | 4 | 2 | Bent (V-shaped) | $H_2O$ | | 5 | 0 | Trigonal Bipyramidal| $PCl_5$ | | 5 | 1 | See-Saw | $SF_4$ | | 5 | 2 | T-shaped | $ClF_3$ | | 5 | 3 | Linear | $XeF_2$ | | 6 | 0 | Octahedral | $SF_6$ | | 6 | 1 | Square Pyramidal | $BrF_5$ | | 6 | 2 | Square Planar | $XeF_4$ | ### Valence Bond Theory (VBT) - **Principle:** Covalent bonds are formed by the overlap of atomic orbitals containing unpaired electrons of opposite spins. - **Types of Overlap:** - **Sigma ($\sigma$) bond:** Head-on (axial) overlap of orbitals. Stronger. - **Pi ($\pi$) bond:** Sideways (lateral) overlap of unhybridized p-orbitals. Weaker. - **Hybridization:** The mixing of atomic orbitals of slightly different energies to form new, equivalent hybrid orbitals of equal energy and shape. - **Hybridization Types & Geometry:** | Hybridization | Atomic Orbitals | Geometry | Example | |---------------|-----------------|---------------------|-----------------| | $sp$ | s + p | Linear | $BeCl_2, C_2H_2$| | $sp^2$ | s + 2p | Trigonal Planar | $BF_3, C_2H_4$ | | $sp^3$ | s + 3p | Tetrahedral | $CH_4, NH_3, H_2O$| | $sp^3d$ | s + 3p + d | Trigonal Bipyramidal| $PCl_5$ | | $sp^3d^2$ | s + 3p + 2d | Octahedral | $SF_6$ | ### Molecular Orbital Theory (MOT) - **Principle:** Atomic orbitals combine to form new molecular orbitals (MOs) that belong to the entire molecule. - **LCAO (Linear Combination of Atomic Orbitals):** Atomic orbitals combine constructively (addition) to form bonding MOs and destructively (subtraction) to form anti-bonding MOs. - **Energy Level Diagram:** - For $O_2, F_2, Ne_2$: $\sigma 1s, \sigma^* 1s, \sigma 2s, \sigma^* 2s, \sigma 2p_z, (\pi 2p_x = \pi 2p_y), (\pi^* 2p_x = \pi^* 2p_y), \sigma^* 2p_z$ - For $B_2, C_2, N_2$: $\sigma 1s, \sigma^* 1s, \sigma 2s, \sigma^* 2s, (\pi 2p_x = \pi 2p_y), \sigma 2p_z, (\pi^* 2p_x = \pi^* 2p_y), \sigma^* 2p_z$ - **Bond Order:** $BO = \frac{1}{2} (N_b - N_a)$ - $N_b$: Number of electrons in bonding MOs. - $N_a$: Number of electrons in anti-bonding MOs. - $BO > 0$ means stable molecule. Higher BO = stronger bond. - **Magnetic Properties:** - **Paramagnetic:** Contains unpaired electrons (attracted to magnetic field). - **Diamagnetic:** All electrons are paired (repelled by magnetic field). - **Examples:** - $O_2$: BO = 2, Paramagnetic (2 unpaired electrons in $\pi^* 2p$ MOs). - $N_2$: BO = 3, Diamagnetic. - $H_2$: BO = 1, Diamagnetic. ### Polarity and Dipole Moment - **Electronegativity:** Tendency of an atom to attract shared electron pair towards itself in a covalent bond. - **Polar Covalent Bond:** Formed between atoms with significant electronegativity difference. Creates partial positive ($\delta^+$) and partial negative ($\delta^-$) charges. - **Non-Polar Covalent Bond:** Formed between atoms with similar electronegativity or identical atoms. - **Dipole Moment ($\mu$):** Measure of the polarity of a molecule. $\mu = q \times d$ - $q$: Magnitude of charge. - $d$: Distance between charges. - Unit: Debye (D). - **Molecular Polarity:** - A molecule with polar bonds can be overall non-polar if the bond dipoles cancel out due to symmetrical geometry (e.g., $CO_2$, $CCl_4$). - A molecule is polar if it contains polar bonds and has an asymmetrical geometry (e.g., $H_2O, NH_3$). ### Hydrogen Bonding - **Definition:** An attractive force between a hydrogen atom covalently bonded to a highly electronegative atom (F, O, N) and another electronegative atom in the same or different molecule. - **Conditions:** 1. Presence of a H atom covalently bonded to F, O, or N. 2. Presence of another highly electronegative atom (F, O, N) with a lone pair. - **Types:** - **Intermolecular H-bonding:** Between different molecules (e.g., $H_2O, HF, NH_3$, alcohols). Increases boiling point, viscosity, surface tension. - **Intramolecular H-bonding:** Within the same molecule (e.g., o-nitrophenol, salicylaldehyde). Decreases boiling point, increases volatility. - **Significance:** Explains high boiling point of water, ice floating on water, structure of proteins and DNA. ### Metallic Bonding - **Electron Sea Model:** Metals consist of positive metal ions immersed in a "sea" of delocalized electrons. - **Properties Explained:** - **High electrical and thermal conductivity:** Due to mobile electrons. - **Lustre:** Due to interaction of light with mobile electrons. - **Malleability and ductility:** Metal ions can slide past each other without breaking the metallic bond. - **High tensile strength:** Strong attractive forces.