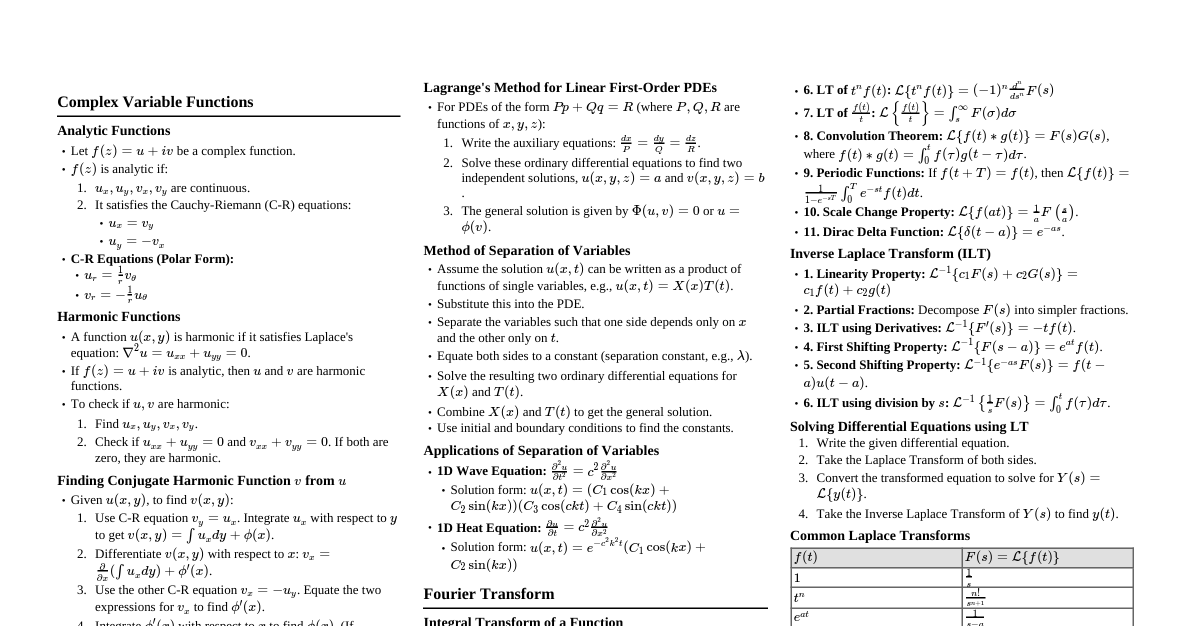
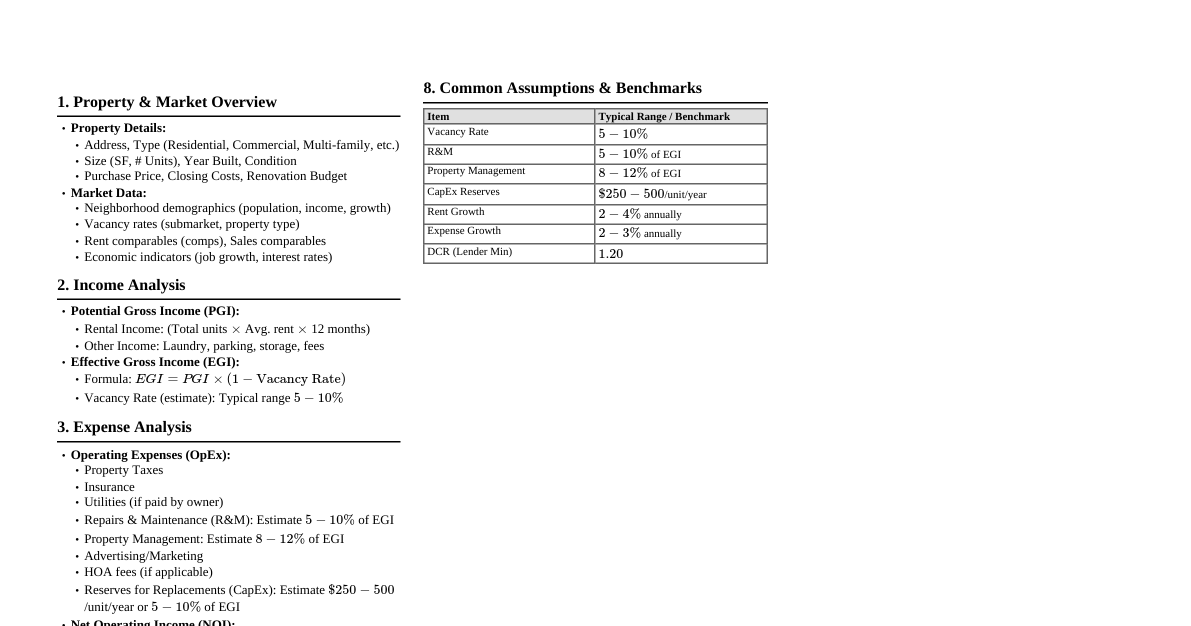
Qualitative Analysis of Organic Compounds Detects the presence of elements like C, H, N, S, halogens, and P. Detection of Carbon and Hydrogen Method: Heating with copper(II) oxide ($CuO$). Carbon: Oxidized to $CO_2$, detected by turning lime water turbid ($Ca(OH)_2 \rightarrow CaCO_3$). Hydrogen: Oxidized to $H_2O$, detected by turning anhydrous copper sulphate blue ($CuSO_4 \rightarrow CuSO_4 \cdot 5H_2O$). Lassaigne's Test (for N, S, Halogens) Converts elements from covalent to ionic form by fusing with sodium metal. Reactions: $Na + C + N \xrightarrow{\Delta} NaCN$ $2Na + S \xrightarrow{\Delta} Na_2S$ $Na + X \xrightarrow{\Delta} NaX$ ($X = Cl, Br, I$) Sodium Fusion Extract: Fused mass boiled with distilled water. Note: If N or S are present, the extract is first boiled with concentrated $HNO_3$ to decompose $NaCN$ or $Na_2S$ to prevent interference. Test for Nitrogen: Extract + $FeSO_4$ + conc. $H_2SO_4 \rightarrow$ Prussian blue color. Indicates formation of $Fe_4[Fe(CN)_6]_3 \cdot xH_2O$. If both N and S are present, $NaSCN$ is formed, giving blood red color with $Fe^{3+}$ ($Fe^{3+} + SCN^- \rightarrow [Fe(SCN)]^{2+}$). Test for Sulphur: Extract + acetic acid + lead acetate $\rightarrow$ black precipitate ($PbS$). Extract + sodium nitroprusside $\rightarrow$ violet color ($[Fe(CN)_5NOS]^{4-}$). Test for Halogens: Extract + $HNO_3$ + $AgNO_3 \rightarrow$ precipitate ($AgX$). $AgCl$: white ppt, soluble in $NH_4OH$. $AgBr$: yellowish ppt, sparingly soluble in $NH_4OH$. $AgI$: yellow ppt, insoluble in $NH_4OH$. Test for Phosphorus Method: Organic compound heated with oxidizing agent ($Na_2O_2$) to oxidize P to phosphoric acid ($H_3PO_4$). $H_3PO_4$ + $NH_3$ + ammonium molybdate $\rightarrow$ yellow coloration or precipitate ($ (NH_4)_3PO_4 \cdot 12MoO_3$). Quantitative Analysis of Organic Compounds Determines the mass percentage of elements. Carbon and Hydrogen Method: Known mass of compound burnt in excess $O_2$ and $CuO$. $C \rightarrow CO_2$, $H \rightarrow H_2O$. $H_2O$ absorbed by anhydrous $CaCl_2$. $CO_2$ absorbed by $KOH$ solution. Calculations: $\%C = \frac{12 \times m_2 \times 100}{44 \times m}$ $\%H = \frac{2 \times m_1 \times 100}{18 \times m}$ where $m$ = mass of organic compound, $m_1$ = mass of $H_2O$, $m_2$ = mass of $CO_2$. Nitrogen Dumas Method: Compound heated with $CuO$ in $CO_2$ atmosphere. $N \rightarrow N_2$. $N_2$ collected over $KOH$ solution (absorbs $CO_2$). Calculation: $\%N = \frac{28 \times V_{STP} \times 100}{22400 \times m}$. Kjeldahl's Method: Compound heated with conc. $H_2SO_4$. $N \rightarrow (NH_4)_2SO_4$. $(NH_4)_2SO_4$ + $NaOH \rightarrow NH_3$. $NH_3$ absorbed in excess standard $H_2SO_4$. Unreacted $H_2SO_4$ titrated with $NaOH$. Calculation: $\%N = \frac{1.4 \times M \times 2(V - V_1/2)}{m}$. Not applicable for: nitro, azo, and ring nitrogen compounds. Halogens (Carius Method) Compound heated with fuming $HNO_3$ and $AgNO_3$ in Carius tube. $X \rightarrow AgX$. $AgX$ filtered, washed, dried, weighed. Calculation: $\%X = \frac{\text{atomic mass of X} \times m_1 \times 100}{\text{molecular mass of AgX} \times m}$. Sulphur Compound heated with $Na_2O_2$ or fuming $HNO_3$. $S \rightarrow H_2SO_4$. $H_2SO_4$ precipitated as $BaSO_4$ by $BaCl_2$. $BaSO_4$ filtered, washed, dried, weighed. Calculation: $\%S = \frac{32 \times m_1 \times 100}{233 \times m}$. Phosphorus Compound heated with fuming $HNO_3$. $P \rightarrow H_3PO_4$. $H_3PO_4$ precipitated as ammonium phosphomolybdate or $Mg_2P_2O_7$. Calculation: (for $Mg_2P_2O_7$) $\%P = \frac{62 \times m_1 \times 100}{222 \times m}$. Oxygen Usually determined by difference ($100\%$ - sum of other elements' percentages). Direct method: Compound heated in $N_2$ stream. $O \rightarrow CO$. $CO$ reacted with $I_2O_5 \rightarrow CO_2$. Calculation: $\%O = \frac{32 \times m_1 \times 100}{88 \times m}$. Methods of Purification of Organic Compounds Based on differences in physical properties (solubility, boiling point, etc.). Sublimation Principle: Solid changes directly to vapor without passing through liquid state. Use: Separates sublimable compounds from non-sublimable impurities. Crystallisation Principle: Differences in solubility of compound and impurities in a solvent at different temperatures. Compound sparingly soluble at room temp, appreciably soluble at higher temp. Cooling a saturated hot solution yields pure crystals. Distillation Principle: Differences in boiling points. Vapors cooled and collected. Simple Distillation: For separating volatile liquids from non-volatile impurities, or liquids with significant boiling point differences. Fractional Distillation: For liquids with small boiling point differences. Uses a fractionating column to increase surface area for heat exchange, enriching vapors in more volatile component. Distillation under Reduced Pressure: For liquids with very high boiling points or those that decompose at/below boiling point. Lowering pressure reduces boiling point. Steam Distillation: For steam volatile and water immiscible substances. Liquid boils below its normal boiling point when sum of vapor pressures ($p_1 + p_2$) equals atmospheric pressure. Differential Extraction Principle: Differences in solubility in two immiscible solvents (one aqueous, one organic). Compound extracted from aqueous medium into an organic solvent where it is more soluble. Continuous Extraction: Used when the organic compound is less soluble in the organic solvent, solvent is repeatedly used. Chromatography Principle: Separation based on differential movement of components of a mixture between stationary and mobile phases. Adsorption Chromatography: Differences in adsorption onto an adsorbent (stationary phase: silica gel, alumina). Column Chromatography: Mixture applied to top of adsorbent column, eluant flows down. Components separate based on adsorption strength. Thin Layer Chromatography (TLC): Mixture spotted on an adsorbent-coated plate. Eluant rises by capillary action, separating components. $R_f$ value used to identify compounds ($R_f = \frac{\text{distance moved by substance}}{\text{distance moved by solvent}}$). Partition Chromatography: Differences in partitioning between stationary (water trapped in paper) and mobile phase (solvent). Paper Chromatography: Mixture spotted on chromatography paper, solvent rises, separating components.