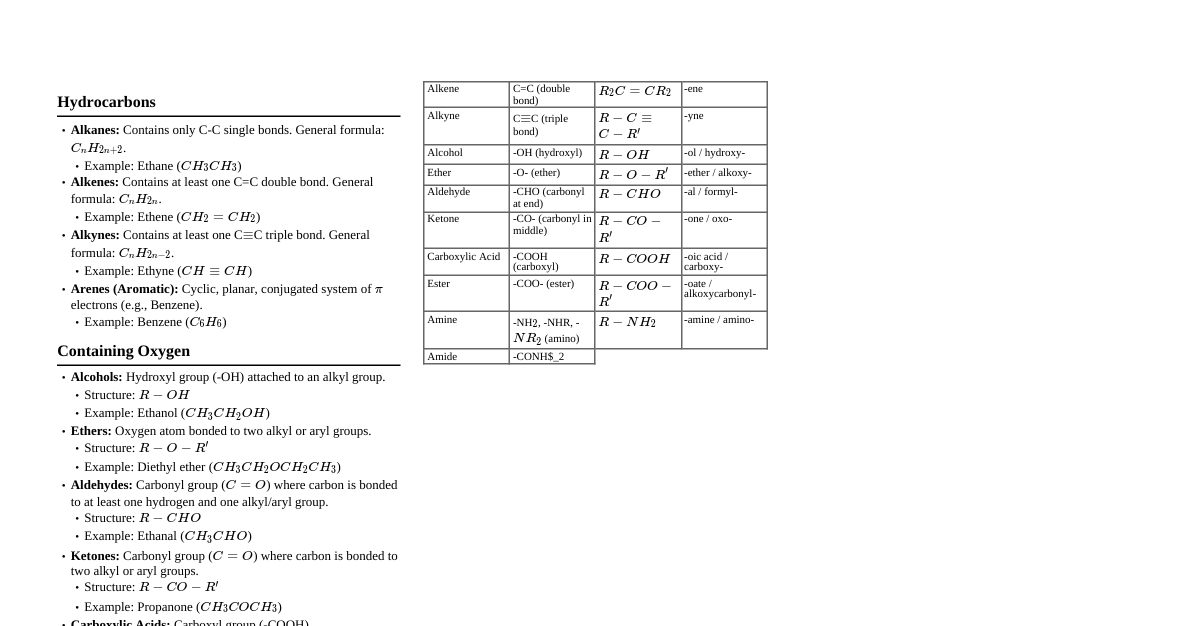
### Basics & Nomenclature #### 1. Hybridization & Geometry - **$sp^3$**: Single bonds, tetrahedral, 109.5° (e.g., alkanes) - **$sp^2$**: One double bond, trigonal planar, 120° (e.g., alkenes, carbonyls) - **$sp$**: One triple bond or two double bonds, linear, 180° (e.g., alkynes, nitriles, allenes) - **Bond Length**: Triple Double > Single #### 2. Inductive Effect (I-effect) - **Definition**: Electron displacement along a sigma bond due to electronegativity difference. - **+I effect (electron-donating)**: Alkyl groups ($CH_3-$, $CH_3CH_2-$ etc.), $COO^-$. Increases electron density. - **-I effect (electron-withdrawing)**: $-NO_2$, $-CN$, $-F$, $-Cl$, $-Br$, $-I$, $-COOH$, $-OR$, $-OH$, $-NH_2$. Decreases electron density. - **Effect on Acidity**: -I effect increases acidity, +I effect decreases acidity. - **Effect on Basicity**: -I effect decreases basicity, +I effect increases basicity. - **Distance dependent**: Decreases rapidly with distance. #### 3. Mesomeric Effect (M-effect) / Resonance Effect (R-effect) - **Definition**: Delocalization of $\pi$-electrons or lone pair electrons via conjugation. - **+M effect (electron-donating)**: Groups with lone pairs ($-\ddot{N}H_2$, $-\ddot{O}H$, $-\ddot{O}R$, $-\ddot{X}$ (halogens)), $C=C$. Increases electron density in conjugated system. - **-M effect (electron-withdrawing)**: Groups with $\pi$-bonds to more electronegative atom ($-NO_2$, $-CN$, $-CHO$, $-COR$, $-COOH$, $-COOR$). Decreases electron density. - **Always effective**: Takes precedence over Inductive effect if both are present and in opposition (except for halogens where -I > +M). #### 4. Hyperconjugation (No-bond Resonance) - **Definition**: Delocalization of $\sigma$-electrons of C-H bond with adjacent empty p-orbital (carbocation), $\pi$-bond (alkene/alkyne), or radical. - **Stability**: More $\alpha$-hydrogens = more hyperconjugation = more stable. - Carbocations: $3^\circ > 2^\circ > 1^\circ > CH_3^+$ - Alkenes: More substituted alkenes are more stable. - Alkyl Radicals: $3^\circ > 2^\circ > 1^\circ > CH_3^\cdot$ #### 5. Aromaticity (Hückel's Rule) - **Conditions**: 1. Cyclic 2. Planar 3. Fully conjugated (every atom in ring has p-orbital) 4. $(4n+2)\pi$ electrons (aromatic) or $(4n)\pi$ electrons (anti-aromatic) - **Examples**: Benzene (6$\pi$, aromatic), Cyclobutadiene (4$\pi$, anti-aromatic), Cyclooctatetraene (8$\pi$, non-aromatic, non-planar). - **Non-aromatic**: If any condition fails (e.g., non-planar, not fully conjugated). #### 6. IUPAC Nomenclature - **Prefix-Word Root-Suffix** - **Prefix**: Substituents (methyl, ethyl, chloro, bromo, nitro, etc.) - **Word Root**: Number of carbons in longest continuous chain (meth, eth, prop, but, pent, hex, hept, oct, non, dec) - **Primary Suffix**: Saturation (-ane, -ene, -yne) - **Secondary Suffix**: Functional group (-ol, -al, -one, -oic acid, -amine, etc.) - **Priority of Functional Groups**: Carboxylic acid > Sulfonic acid > Ester > Acid halide > Amide > Nitrile > Aldehyde > Ketone > Alcohol > Amine > Alkene > Alkyne > Alkane > Ether > Halogen > Nitro. - **Cyclic compounds**: Use 'cyclo' prefix. - **Bicyclo/Spiro compounds**: Specific rules apply. ### Isomerism #### 1. Structural Isomerism (Constitutional) - **Definition**: Same molecular formula, different connectivity. - **Chain Isomerism**: Different carbon skeletons (e.g., n-pentane, isopentane, neopentane). - **Position Isomerism**: Different position of functional group or substituent (e.g., 1-chloropropane, 2-chloropropane). - **Functional Group Isomerism**: Different functional groups (e.g., ethanol & dimethylether). - **Metamerism**: Different alkyl groups around a polyvalent functional group (e.g., diethyl ether, methyl propyl ether). - **Tautomerism**: Special type of functional isomerism where isomers exist in dynamic equilibrium, usually involving migration of a proton and a $\pi$-bond (e.g., keto-enol tautomerism). Enol form is stable if conjugated/aromatic. #### 2. Stereoisomerism - **Definition**: Same molecular formula and connectivity, different spatial arrangement. - **Conformational Isomerism**: Interconvertible by rotation around single bonds. - **Alkanes**: Newman projections (e.g., ethane - staggered is more stable than eclipsed). - **Cyclohexane**: Chair, boat, twist-boat forms. Chair is most stable. Axial/equatorial positions. - **Configurational Isomerism**: Cannot be interconverted without breaking bonds. - **Geometrical (cis-trans) Isomerism**: Due to restricted rotation (double bonds, cyclic structures). - **Alkenes**: Cis (same side), Trans (opposite side). Trans is generally more stable due to less steric hindrance. E/Z nomenclature for complex alkenes. - **Cyclic Compounds**: Cis/trans for disubstituted rings. - **Optical Isomerism (Enantiomerism)**: Due to chiral center (asymmetric carbon). - **Chiral Center**: Carbon bonded to four different groups. - **Enantiomers**: Non-superimposable mirror images. Have identical physical and chemical properties (except rotation of plane-polarized light and reaction with other chiral molecules). - **Diastereomers**: Stereoisomers that are not mirror images. Have different physical and chemical properties. - **Meso Compounds**: Contain chiral centers but are achiral due to internal plane of symmetry. Do not rotate plane-polarized light. - **Specific Rotation**: $[\alpha]_D = \frac{\alpha}{l \times c}$ (observed rotation / path length x concentration). - **Racemic Mixture**: Equimolar mixture of enantiomers. Optically inactive (external compensation). - **Resolution**: Separation of enantiomers from a racemic mixture. - **R/S Configuration**: Cahn-Ingold-Prelog priority rules. 1. Assign priority based on atomic number. 2. Orient molecule so lowest priority group is pointing away. 3. Trace path from 1-2-3. Clockwise = R, Counter-clockwise = S. ### Reaction Mechanisms #### 1. Reaction Intermediates - **Carbocations**: $sp^2$ hybridized, trigonal planar, electron deficient (6 e-). Stability: $3^\circ > 2^\circ > 1^\circ > CH_3^+$. Stabilized by +I, +M, hyperconjugation. Undergo rearrangements (hydride/alkyl shifts) to form more stable carbocations. - **Carbanions**: $sp^3$ hybridized, pyramidal, electron rich (8 e-). Stability: $CH_3^- > 1^\circ > 2^\circ > 3^\circ$. Stabilized by -I, -M. - **Free Radicals**: $sp^2$ hybridized, trigonal planar or pyramidal, one unpaired electron (7 e-). Stability: $3^\circ > 2^\circ > 1^\circ > CH_3^\cdot$. Stabilized by +I, hyperconjugation, resonance. - **Carbenes**: Divalent carbon, $sp^2$ (singlet) or $sp$ (triplet). Electrophilic. #### 2. Reaction Types - **Substitution**: Atom/group replaced by another. - **Nucleophilic Substitution ($S_N1$, $S_N2$)**: - **$S_N1$**: Unimolecular, 2 steps, carbocation intermediate. Rate = k[substrate]. Favored by $3^\circ$ alkyl halides, polar protic solvents. Racemization + inversion. - **$S_N2$**: Bimolecular, 1 step (concerted), transition state. Rate = k[substrate][nucleophile]. Favored by $1^\circ$ alkyl halides, strong nucleophiles, polar aprotic solvents. Inversion of configuration (Walden inversion). - **Electrophilic Substitution ($S_E$)**: Characteristic of aromatic compounds (e.g., nitration, halogenation, sulfonation, Friedel-Crafts alkylation/acylation). - **Free Radical Substitution ($S_R$)**: Alkanes with halogens in presence of light/heat. - **Addition**: Atoms/groups added across a $\pi$-bond. - **Electrophilic Addition ($A_E$)**: Alkenes/Alkynes. Markovnikov's rule (H adds to carbon with more H's). Anti-Markovnikov's with peroxides for HBr. - **Nucleophilic Addition ($A_N$)**: Aldehydes/Ketones. - **Free Radical Addition ($A_R$)**: (e.g., HBr to alkene in presence of peroxide). - **Elimination ($E1$, $E2$)**: Removal of atoms/groups to form a $\pi$-bond. - **$E1$**: Unimolecular, 2 steps, carbocation intermediate. Rate = k[substrate]. Favored by $3^\circ$ alkyl halides, polar protic solvents, weak bases. Saytzeff's rule (more substituted alkene is major product). - **$E2$**: Bimolecular, 1 step (concerted). Rate = k[substrate][base]. Favored by $1^\circ/2^\circ/3^\circ$ alkyl halides, strong bases, polar aprotic solvents. Anti-periplanar geometry required for leaving group and $\beta$-hydrogen. Saytzeff's rule (unless bulky base). - **Rearrangement**: Atoms/groups migrate within a molecule. - **Redox**: Oxidation/Reduction reactions. #### 3. Important Reagents & Catalysts - **Oxidizing Agents**: $KMnO_4$ (cold/dilute/alkaline for diols, hot/acidic for cleavage), $K_2Cr_2O_7/H^+$, $CrO_3$, PCC, Jones Reagent, $O_3$ (ozonolysis). - **Reducing Agents**: $H_2/Ni, Pd, Pt$ (catalytic hydrogenation), $LiAlH_4$, $NaBH_4$, Red P + HI, Zn-Hg/HCl (Clemmensen), $N_2H_4/KOH$ (Wolff-Kishner). - **Lewis Acids**: $AlCl_3$, $FeCl_3$, $BF_3$, $ZnCl_2$. - **Bases**: $NaOH$, $KOH$, $NaNH_2$, $t-BuOK$, $LDA$, Pyridine. ### Alkanes, Alkenes & Alkynes #### 1. Alkanes - **General Formula**: $C_nH_{2n+2}$ - **Preparation**: - Hydrogenation of alkenes/alkynes ($H_2/Ni, Pd, Pt$). - Wurtz Reaction: $2RX + 2Na \xrightarrow{dry \ ether} R-R + 2NaX$ (for symmetrical alkanes). - Decarboxylation of carboxylic acids: $RCOONa + NaOH \xrightarrow{CaO, \Delta} RH + Na_2CO_3$. - Kolbe's Electrolytic Method: $2RCOONa \xrightarrow{electrolysis} R-R + 2CO_2 + 2NaOH + H_2$. - Reduction of alkyl halides: $RX \xrightarrow{Zn/HCl \ or \ LiAlH_4} RH$. - **Reactions**: - **Free Radical Halogenation**: $CH_4 + Cl_2 \xrightarrow{hv} CH_3Cl + HCl$. Reactivity of H: $3^\circ > 2^\circ > 1^\circ$. Selectivity of Halogen: $Br_2$ is more selective than $Cl_2$. - **Combustion**: $C_nH_{2n+2} + (\frac{3n+1}{2})O_2 \rightarrow nCO_2 + (n+1)H_2O$. - **Pyrolysis/Cracking**: Thermal decomposition at high temp. #### 2. Alkenes - **General Formula**: $C_nH_{2n}$ - **Preparation**: - **Dehydration of Alcohols**: $R-CH_2-CH_2-OH \xrightarrow{conc. \ H_2SO_4, \Delta} R-CH=CH_2 + H_2O$. Saytzeff's rule. - **Dehydrohalogenation of Alkyl Halides**: $R-CH_2-CH_2-X \xrightarrow{alc. \ KOH, \Delta} R-CH=CH_2 + HX$. Saytzeff's rule. - **Dehalogenation of Vicinal Dihalides**: $R-CHBr-CHBr-R' \xrightarrow{Zn \ dust} R-CH=CH-R' + ZnBr_2$. - **Partial Hydrogenation of Alkynes**: - **Lindlar's Catalyst** ($Pd/BaSO_4 + quinoline$): Alkyne $\rightarrow$ Cis-alkene. - **Birch Reduction** ($Na/liq. \ NH_3$): Alkyne $\rightarrow$ Trans-alkene. - **Reactions**: - **Addition Reactions**: - **Hydrogenation**: $H_2/Ni, Pd, Pt \rightarrow$ Alkane (Syn addition). - **Halogenation**: $X_2/CCl_4 \rightarrow$ Vicinal dihalide (Anti addition). - **Hydrohalogenation**: $HX \rightarrow$ Alkyl halide (Markovnikov's Rule). $HBr/peroxide \rightarrow$ Anti-Markovnikov's addition. - **Hydration**: $H_2O/H_2SO_4 \rightarrow$ Alcohol (Markovnikov's Rule, via carbocation). - **Oxymercuration-Demercuration**: $Hg(OAc)_2, H_2O / NaBH_4 \rightarrow$ Alcohol (Markovnikov's, no rearrangement). - **Hydroboration-Oxidation**: $BH_3 \cdot THF / H_2O_2, OH^- \rightarrow$ Alcohol (Anti-Markovnikov's, Syn addition). - **Oxidation**: - **Baeyer's Reagent** (cold, dilute, alkaline $KMnO_4$): Alkene $\rightarrow$ Vicinal Diol (Syn addition). - **Hot, acidic $KMnO_4$ or $K_2Cr_2O_7$**: Cleavage of alkene. - **Ozonolysis**: $O_3/Zn, H_2O$: Cleavage to aldehydes/ketones. - **Epoxidation**: $RCO_3H \rightarrow$ Epoxide. - **Polymerization**: $n(CH_2=CH_2) \rightarrow -(CH_2-CH_2)_n-$ (polyethylene). #### 3. Alkynes - **General Formula**: $C_nH_{2n-2}$ - **Preparation**: - **Dehydrohalogenation of Vicinal/Geminal Dihalides**: $R-CHX-CHX-R' \xrightarrow{alc. \ KOH} R-CX=CH-R' \xrightarrow{NaNH_2} R-C \equiv C-R'$. - **From Calcium Carbide**: $CaC_2 + 2H_2O \rightarrow CH \equiv CH + Ca(OH)_2$. - **Terminal alkynes from alkyl halides** (via acetylide anion): $RC \equiv CNa + R'X \rightarrow RC \equiv CR' + NaX$. - **Reactions**: - **Acidity of Terminal Alkynes**: $R-C \equiv C-H$ are weakly acidic, react with strong bases ($NaNH_2$) to form acetylides. - **Addition Reactions**: Similar to alkenes but occur twice. - **Hydrogenation**: $H_2/Ni, Pd, Pt \rightarrow$ Alkane. - **Partial Hydrogenation**: Lindlar's $\rightarrow$ Cis-alkene, Birch $\rightarrow$ Trans-alkene. - **Halogenation**: $X_2 \rightarrow$ Tetrahalide (via dihalide). - **Hydrohalogenation**: $HX \rightarrow$ Geminal dihalide (Markovnikov's). - **Hydration**: $HgSO_4/H_2SO_4 \rightarrow$ Ketone (via enol tautomerization, Markovnikov's). For acetylene, acetaldehyde. - **Oxidation**: - **Hot, acidic $KMnO_4$**: Cleavage to carboxylic acids. - **Ozonolysis**: $O_3/H_2O \rightarrow$ Carboxylic acids. - **Cyclic Polymerization**: $3CH \equiv CH \xrightarrow{Red \ Hot \ Iron \ Tube} C_6H_6$ (Benzene). ### Haloalkanes & Haloarenes #### 1. Haloalkanes (Alkyl Halides) - **Preparation**: - **From Alcohols**: $ROH + HX \xrightarrow{ZnCl_2 \ (Lucas \ Reagent)} RX + H_2O$. $ROH + PCl_5 \rightarrow RCl + POCl_3 + HCl$. $ROH + SOCl_2 \rightarrow RCl + SO_2 + HCl$ (Darzen's process, best method). - **From Alkenes**: $HX$ addition (Markovnikov's), $X_2$ addition (vicinal dihalide), $HBr/peroxide$ (Anti-Markovnikov's). - **Free Radical Halogenation of Alkanes**: $RH + X_2 \xrightarrow{hv} RX + HX$. - **Halogen Exchange**: - **Finkelstein Reaction**: $RCl/RBr + NaI \xrightarrow{Acetone} RI + NaCl/NaBr$. - **Swarts Reaction**: $CH_3Br + AgF \rightarrow CH_3F + AgBr$. - **Reactions**: - **Nucleophilic Substitution ($S_N1, S_N2$)**: - $RX + NaOH \rightarrow ROH$ (alcohol) - $RX + H_2O \rightarrow ROH$ (alcohol) - $RX + NaOR' \rightarrow ROR'$ (ether, Williamson synthesis) - $RX + NaCN \rightarrow RCN$ (nitrile) - $RX + KCN \rightarrow RCN$ (nitrile) - $RX + AgCN \rightarrow RNC$ (isocyanide) - $RX + NaNO_2 \rightarrow R-ONO$ (alkyl nitrite) - $RX + AgNO_2 \rightarrow R-NO_2$ (nitroalkane) - $RX + KSH \rightarrow RSH$ (thiol) - $RX + R'NH_2 \rightarrow RR'NH$ (amine) - $RX + Mg \xrightarrow{dry \ ether} RMgX$ (Grignard Reagent) - **Elimination ($E1, E2$)**: Dehydrohalogenation with alcoholic KOH. - **Reaction with Metals**: - **Wurtz Reaction**: $2RX + 2Na \xrightarrow{dry \ ether} R-R + 2NaX$. - **Frankland Reaction**: $2RX + Zn \rightarrow R-R + ZnX_2$. - **Reduction**: $RX \xrightarrow{Zn/HCl} RH$. #### 2. Haloarenes (Aryl Halides) - **Preparation**: - **Electrophilic Substitution of Benzene**: $C_6H_6 + Cl_2 \xrightarrow{FeCl_3} C_6H_5Cl + HCl$. - **Sandmeyer Reaction**: $ArN_2^+Cl^- \xrightarrow{Cu_2X_2/HX} ArX + N_2$. - **Gattermann Reaction**: $ArN_2^+Cl^- \xrightarrow{Cu/HX} ArX + N_2$. - **Balz-Schiemann Reaction**: $ArN_2^+BF_4^- \xrightarrow{\Delta} ArF + BF_3 + N_2$. - **Hunsdiecker Reaction**: $ArCOOAg + Br_2 \xrightarrow{CCl_4} ArBr + AgBr + CO_2$. - **Reactions**: - **Low Reactivity towards Nucleophilic Substitution**: Due to partial double bond character of C-X bond (resonance) and $sp^2$ hybridized carbon. - **Nucleophilic Substitution (Activated)**: With electron-withdrawing groups (e.g., $-NO_2$) at ortho/para positions, $S_NAr$ reaction occurs (e.g., G.N.D.A. reaction). - **Electrophilic Substitution**: Halogen is ortho/para directing and deactivating ($Cl, Br, I$). - **Reaction with Metals**: - **Wurtz-Fittig Reaction**: $ArX + RX + 2Na \xrightarrow{dry \ ether} Ar-R + 2NaX$. - **Fittig Reaction**: $2ArX + 2Na \xrightarrow{dry \ ether} Ar-Ar + 2NaX$. - **Ullmann Reaction**: $2ArI \xrightarrow{Cu, \Delta} Ar-Ar + CuI_2$. ### Alcohols, Phenols & Ethers #### 1. Alcohols - **Preparation**: - **From Alkenes**: Hydration ($H_2O/H_2SO_4$), Hydroboration-Oxidation, Oxymercuration-Demercuration. - **From Carbonyl Compounds**: - Reduction of aldehydes/ketones ($LiAlH_4, NaBH_4, H_2/Ni$). - Grignard Reagent (RMgX) with aldehydes/ketones: - HCHO $\rightarrow 1^\circ$ alcohol - RCHO $\rightarrow 2^\circ$ alcohol - RCOR' $\rightarrow 3^\circ$ alcohol - **From Carboxylic Acids/Esters**: Reduction with $LiAlH_4$ (not $NaBH_4$). - **From Primary Amines**: $RNH_2 + HNO_2 \rightarrow ROH + N_2 + H_2O$. - **Reactions**: - **Acidity**: $CH_3OH > 1^\circ > 2^\circ > 3^\circ$. Weaker acids than water. - **Reaction with active metals**: $2ROH + 2Na \rightarrow 2RONa + H_2$. - **Esterification**: $ROH + R'COOH \xrightarrow{H^+} R'COOR + H_2O$. - **Reaction with HX**: $ROH + HX \rightarrow RX + H_2O$. (Lucas test: $ZnCl_2 + HCl$). Reactivity of ROH: $3^\circ > 2^\circ > 1^\circ$. - **Reaction with $PCl_5, PCl_3, SOCl_2$**: $\rightarrow RX$. - **Dehydration**: $ROH \xrightarrow{conc. \ H_2SO_4, \Delta} Alkene$ (at high temp), $Ether$ (at low temp, intermolecular). Saytzeff's rule for alkene. - **Oxidation**: - $1^\circ ROH \xrightarrow{PCC \ or \ CrO_3 \ in \ anhydrous \ medium} Aldehyde$. - $1^\circ ROH \xrightarrow{strong \ oxidizing \ agent} Carboxylic \ acid$. - $2^\circ ROH \xrightarrow{oxidizing \ agent} Ketone$. - $3^\circ ROH$: Resistant to oxidation under normal conditions; vigorous conditions lead to C-C bond cleavage. - **Distinction tests**: Lucas test ($3^\circ$ immediate turbidity, $2^\circ$ after 5-10 min, $1^\circ$ no turbidity at RT). Victor Meyer test. #### 2. Phenols - **Preparation**: - **From Haloarenes**: $C_6H_5Cl + NaOH \xrightarrow{623K, \ 300atm} C_6H_5ONa \xrightarrow{H^+} C_6H_5OH$ (Dow's process). - **From Benzene Sulfonic Acid**: $C_6H_5SO_3H + NaOH \xrightarrow{\Delta} C_6H_5ONa \xrightarrow{H^+} C_6H_5OH$. - **From Diazo Salts**: $ArN_2^+Cl^- + H_2O \xrightarrow{\Delta} ArOH + N_2 + HCl$. - **From Cumene**: Cumene $\xrightarrow{O_2} \text{Cumene Hydroperoxide} \xrightarrow{H_2SO_4} Phenol + Acetone$. - **Reactions**: - **Acidity**: Phenols > Alcohols. Electron-withdrawing groups (e.g., $-NO_2$) increase acidity, electron-donating groups (e.g., $-CH_3$) decrease acidity. $p$-nitrophenol is more acidic than $o$-nitrophenol due to inter/intra-molecular H-bonding. - **Electrophilic Aromatic Substitution**: -OH is ortho/para directing and activating. - **Nitration**: Dilute $HNO_3 \rightarrow o-/p$-nitrophenol. Conc. $HNO_3 \rightarrow$ Picric acid (2,4,6-trinitrophenol). - **Halogenation**: $Br_2/CS_2 \rightarrow p$-bromophenol. $Br_2/H_2O \rightarrow$ 2,4,6-tribromophenol (white ppt). - **Sulfonation**: Conc. $H_2SO_4 \rightarrow o-/p$-phenolsulfonic acid (temp dependent). - **Reimer-Tiemann Reaction**: Phenol $\xrightarrow{CHCl_3/NaOH} Salicylaldehyde$. - **Kolbe's Reaction**: Phenol $\xrightarrow{CO_2/NaOH} Salicylic \ acid$. - **Friedel-Crafts Reaction**: Difficult due to complexation with Lewis acid. - **Oxidation**: With $K_2Cr_2O_7 \rightarrow$ Benzoquinone. - **Reaction with Zinc Dust**: Phenol $\xrightarrow{Zn \ dust} Benzene$. - **Ferric Chloride Test**: Phenols give characteristic coloration (violet, green, etc.) with neutral $FeCl_3$. #### 3. Ethers - **Preparation**: - **Williamson Synthesis**: $RX + R'ONa \rightarrow ROR' + NaX$. Best for $1^\circ$ alkyl halides. If $3^\circ$ alkyl halide is used, elimination (alkene) is major product. - **Dehydration of Alcohols**: $2ROH \xrightarrow{conc. \ H_2SO_4, \Delta \ (140^\circ C)} ROR + H_2O$. (Intramolecular dehydration at $170^\circ C$ gives alkene). - **Reactions**: - **Cleavage by HI/HBr**: $ROR' + HI \rightarrow RI + R'OH$. If excess HI, then $ROR' + 2HI \rightarrow RI + R'I + H_2O$. Reactivity of HX: $HI > HBr > HCl$. Forms alkyl halide with smaller alkyl group if $1^\circ/2^\circ$. If one group is $3^\circ$, then $3^\circ$ carbocation forms, giving $3^\circ$ halide. - **Electrophilic Substitution (Aromatic Ethers)**: -OR is ortho/para directing and activating. - **Peroxide Formation**: Ethers react with atmospheric oxygen to form explosive peroxides. ### Carbonyl Compounds (Aldehydes & Ketones) #### 1. Preparation - **Oxidation of Alcohols**: - $1^\circ ROH \xrightarrow{PCC} Aldehyde$. - $2^\circ ROH \xrightarrow{PCC \ or \ K_2Cr_2O_7/H^+} Ketone$. - **Ozonolysis of Alkenes**: Alkene $\xrightarrow{O_3/Zn, H_2O} Aldehydes/Ketones$. - **Hydration of Alkynes**: Alkyne $\xrightarrow{HgSO_4/H_2SO_4} Aldehyde \ (acetylene) \ or \ Ketone \ (other \ alkynes)$. - **From Acid Chlorides**: - **Rosenmund Reduction**: $RCOCl + H_2 \xrightarrow{Pd/BaSO_4} RCHO + HCl$. - **With Dialkylcadmium**: $2RCOCl + R'_2Cd \rightarrow 2RCOR' + CdCl_2$. - **From Nitriles**: - **Stephen Reaction**: $RCN + SnCl_2/HCl \rightarrow RCH=NH \cdot HCl \xrightarrow{H_3O^+} RCHO$. - **With DIBAL-H**: $RCN \xrightarrow{DIBAL-H \ (low \ temp)} RCH=NH \xrightarrow{H_3O^+} RCHO$. - **Grignard Reagent**: $RCN + R'MgX \xrightarrow{H_3O^+} RCOR'$. - **Aromatic Aldehydes**: - **Etard Reaction**: Toluene $\xrightarrow{CrO_2Cl_2} \text{Chromium complex} \xrightarrow{H_3O^+} Benzaldehyde$. - **Gattermann-Koch Reaction**: Benzene $\xrightarrow{CO, HCl/AlCl_3, CuCl} Benzaldehyde$. - **Gattermann Reaction**: Benzene $\xrightarrow{HCN, HCl/AlCl_3} \text{Imine} \xrightarrow{H_3O^+} Benzaldehyde$. - **Oxidation of Methylbenzene**: Toluene $\xrightarrow{CrO_3/(CH_3CO)_2O} \text{Benzylidene diacetate} \xrightarrow{H_3O^+} Benzaldehyde$. #### 2. Reactions - **Nucleophilic Addition Reactions**: Aldehydes are more reactive than ketones due to steric and electronic reasons. - **Addition of HCN**: $\rightarrow$ Cyanohydrin. - **Addition of $NaHSO_3$**: $\rightarrow$ Bisulfite addition product (crystalline solid, used for purification). - **Addition of Grignard Reagent**: $\rightarrow$ Alcohols. - **Addition of Alcohols**: $RCHO + R'OH \xrightarrow{H^+} Hemiacetal \xrightarrow{R'OH} Acetal$. - **Addition of Ammonia Derivatives**: $(>C=O + H_2N-Z \rightarrow >C=N-Z + H_2O)$. - Hydroxylamine ($NH_2OH$) $\rightarrow$ Oxime. - Hydrazine ($NH_2NH_2$) $\rightarrow$ Hydrazone. - Phenylhydrazine $\rightarrow$ Phenylhydrazone. - 2,4-Dinitrophenylhydrazine (2,4-DNP) $\rightarrow$ 2,4-DNP derivative (yellow/orange ppt, test for carbonyl). - **Reduction Reactions**: - **To Alcohols**: $H_2/Ni, Pd, Pt$ or $LiAlH_4$ or $NaBH_4$. - **To Hydrocarbons**: - **Clemmensen Reduction**: $>C=O \xrightarrow{Zn-Hg/conc. \ HCl} -CH_2-$. - **Wolff-Kishner Reduction**: $>C=O \xrightarrow{N_2H_4, KOH/Ethylene \ Glycol, \Delta} -CH_2-$. - **Oxidation Reactions**: - **Aldehydes**: Easily oxidized to carboxylic acids. - **Tollens' Reagent** ($Ag(NH_3)_2^+OH^-$): Aldehyde $\rightarrow$ Carboxylic acid salt + Silver mirror (test for aldehyde). - **Fehling's Solution** ($Cu^{2+}$ complex): Aldehyde $\rightarrow$ Carboxylic acid salt + Red ppt of $Cu_2O$ (test for aldehyde, not for aromatic aldehydes). - **Benedict's Solution**: Similar to Fehling's. - **Strong oxidizing agents** ($KMnO_4, K_2Cr_2O_7$) also oxidize aldehydes. - **Ketones**: Resistant to oxidation under mild conditions. Vigorous oxidation causes C-C bond cleavage (Popov's Rule). - **Reactions due to $\alpha$-hydrogens**: - **Aldol Condensation**: Carbonyl compound with $\alpha$-H reacts with dilute base to form $\beta$-hydroxy carbonyl compound, which dehydrates to $\alpha,\beta$-unsaturated carbonyl compound. - Cross-Aldol: Between two different carbonyl compounds. - **Haloform Reaction**: Methyl ketones ($CH_3COR$) or $CH_3CH(OH)R$ react with $X_2/NaOH$ to form Haloform ($CHX_3$, yellow ppt for $CHI_3$) and carboxylate. - **Cannizzaro Reaction**: Aldehydes without $\alpha$-H (e.g., HCHO, $C_6H_5CHO$) undergo disproportionation in conc. base to form alcohol and carboxylic acid salt. - **Electrophilic Substitution (Aromatic Aldehydes/Ketones)**: -CHO, -COR are meta-directing and deactivating. ### Carboxylic Acids & Derivatives #### 1. Carboxylic Acids - **Preparation**: - **Oxidation of $1^\circ$ Alcohols/Aldehydes**: $\xrightarrow{KMnO_4, K_2Cr_2O_7, CrO_3}$. - **From Nitriles**: $RCN \xrightarrow{H_3O^+} RCOOH$. - **From Grignard Reagent**: $RMgX + CO_2 \xrightarrow{dry \ ether} RCOOMgX \xrightarrow{H_3O^+} RCOOH$. - **Hydrolysis of Esters**: $RCOOR' \xrightarrow{H_3O^+} RCOOH + R'OH$. - **From Alkylbenzenes**: Toluene $\xrightarrow{KMnO_4/KOH} Benzoic \ Acid$. - **Reactions**: - **Acidity**: Carboxylic acids are stronger acids than phenols and alcohols. - **Factors affecting acidity**: Electron-withdrawing groups (-I, -M) increase acidity, electron-donating groups (+I, +M) decrease acidity. - Form salts with $NaHCO_3$ (effervescence of $CO_2$). - **Formation of Acid Derivatives**: - **Acid Chlorides**: $RCOOH + SOCl_2 \rightarrow RCOCl + SO_2 + HCl$. - **Anhydrides**: $2RCOOH \xrightarrow{P_2O_5, \Delta} (RCO)_2O + H_2O$. - **Esters**: $RCOOH + R'OH \xrightarrow{H^+} RCOOR' + H_2O$ (Esterification). - **Amides**: $RCOOH + NH_3 \xrightarrow{\Delta} RCONH_2 + H_2O$ (via ammonium salt). - **Reduction**: $RCOOH \xrightarrow{LiAlH_4} RCH_2OH$ (not $NaBH_4$). - **Hell-Volhard-Zelinsky (HVZ) Reaction**: $R-CH_2-COOH \xrightarrow{X_2/Red \ P} R-CH(X)-COOH$. - **Decarboxylation**: $RCOOH \xrightarrow{NaOH/CaO, \Delta} RH + Na_2CO_3$. - **Electrophilic Substitution (Aromatic Carboxylic Acids)**: -COOH is meta-directing and deactivating. #### 2. Acid Derivatives - **Relative Reactivity**: Acid Chloride > Anhydride > Ester > Amide (towards nucleophilic acyl substitution). - **Acid Chlorides (RCOCl)**: - **Hydrolysis**: $\rightarrow RCOOH$. - **Alcoholysis**: $\rightarrow RCOOR'$ (ester). - **Ammonolysis**: $\rightarrow RCONH_2$ (amide). - **Reduction**: $\xrightarrow{H_2/Pd/BaSO_4} RCHO$ (Rosenmund), $\xrightarrow{LiAlH_4} RCH_2OH$. - **With Grignard**: $RCOCl + R'MgX \rightarrow Ketone \rightarrow 3^\circ Alcohol$. - **Acid Anhydrides $((RCO)_2O)$**: - Similar reactions to acid chlorides, but less reactive. - **Esters (RCOOR')**: - **Hydrolysis**: $RCOOR' \xrightarrow{H_3O^+} RCOOH + R'OH$ (acidic hydrolysis, reversible). - **Saponification**: $RCOOR' \xrightarrow{NaOH} RCOONa + R'OH$ (basic hydrolysis, irreversible). - **Ammonolysis**: $\rightarrow RCONH_2 + R'OH$. - **Reduction**: $\xrightarrow{LiAlH_4} RCH_2OH + R'OH$. - **With Grignard**: $RCOOR' + R''MgX \rightarrow Ketone \rightarrow 3^\circ Alcohol$. - **Amides (RCONH_2)**: - **Hydrolysis**: $\xrightarrow{H_3O^+} RCOOH + NH_4^+$. - **Reduction**: $\xrightarrow{LiAlH_4} RCH_2NH_2$ (amine). - **Hofmann Bromamide Degradation**: $RCONH_2 + Br_2 + 4NaOH \rightarrow RNH_2 + Na_2CO_3 + 2NaBr + 2H_2O$ (produces primary amine with one less carbon). - **Dehydration**: $RCONH_2 \xrightarrow{P_2O_5, \Delta} RCN$ (nitrile). ### Amines #### 1. Preparation - **Reduction of Nitro Compounds**: $RNO_2 \xrightarrow{Sn/HCl \ or \ Fe/HCl \ or \ H_2/Pd} RNH_2$. - **Ammonolysis of Alkyl Halides**: $RX + NH_3 \rightarrow RNH_2 + HX$. (Can lead to $2^\circ, 3^\circ$ amines and quaternary ammonium salts). - **Gabriel Phthalimide Synthesis**: For $1^\circ$ amines. Phthalimide $\xrightarrow{KOH} \text{Potassium phthalimide} \xrightarrow{RX} \text{N-alkylphthalimide} \xrightarrow{H_3O^+ \ or \ N_2H_4} RNH_2 + \text{Phthalic acid/Hydrazine}$. - **Hofmann Bromamide Degradation**: $RCONH_2 \xrightarrow{Br_2/NaOH} RNH_2$. (Decreases carbon chain by one). - **Reduction of Nitriles**: $RCN \xrightarrow{LiAlH_4 \ or \ H_2/Ni} RCH_2NH_2$. - **Reduction of Amides**: $RCONH_2 \xrightarrow{LiAlH_4} RCH_2NH_2$. #### 2. Reactions - **Basicity**: Amines are basic due to lone pair on Nitrogen. - **Order of Basicity (aqueous)**: $2^\circ > 1^\circ > 3^\circ > NH_3$ (for alkyl amines). - **Order of Basicity (gas phase)**: $3^\circ > 2^\circ > 1^\circ > NH_3$. - **Aromatic amines** (e.g., Aniline) are much less basic than alkyl amines due to resonance delocalization of lone pair. - **Effect of substituents**: Electron-donating groups increase basicity, electron-withdrawing groups decrease basicity. - **Alkylation**: $RNH_2 + RX \rightarrow R_2NH \rightarrow R_3N \rightarrow R_4N^+X^-$. - **Acylation**: $RNH_2 + R'COCl \rightarrow RNHCOR' + HCl$ (Amide formation). - **Carbylamine Reaction (Isocyanide Test)**: $1^\circ$ amine $\xrightarrow{CHCl_3/KOH, \Delta} RNC$ (foul smelling). Used to distinguish $1^\circ$ amines. - **Reaction with Nitrous Acid ($HNO_2$)**: - $1^\circ$ Aliphatic amine: $\xrightarrow{HNO_2} ROH + N_2 + H_2O$ (evolution of $N_2$ gas). - $1^\circ$ Aromatic amine (Aniline): $\xrightarrow{HNO_2/HCl \ (0-5^\circ C)} ArN_2^+Cl^-$ (Diazotization, forms diazonium salt). - $2^\circ$ Amine: $\xrightarrow{HNO_2} N$-nitrosoamine (yellow oily product). - $3^\circ$ Amine: Forms soluble salts. - **Hinsberg Test**: Used to distinguish $1^\circ, 2^\circ, 3^\circ$ amines. - Reagent: Benzene sulfonyl chloride ($C_6H_5SO_2Cl$). - $1^\circ$ Amine: Forms N-alkylbenzenesulfonamide, which is soluble in KOH. - $2^\circ$ Amine: Forms N,N-dialkylbenzenesulfonamide, which is insoluble in KOH. - $3^\circ$ Amine: Does not react with reagent. - **Electrophilic Substitution (Aromatic Amines)**: $-NH_2$ is strong ortho/para activating group. - **Bromination**: Aniline $\xrightarrow{Br_2/H_2O} 2,4,6$-tribromoaniline (white ppt). To get monobrominated product, amino group must be protected by acetylation. - **Nitration**: Direct nitration gives tarry products and $m$-nitroaniline due to anilinium ion formation. Amino group is protected by acetylation. - **Sulfonation**: Aniline $\xrightarrow{conc. \ H_2SO_4} \text{Anilinium Hydrogen Sulphate} \xrightarrow{\Delta} \text{Sulphanilic acid}$ (Zwitterionic form). ### Biomolecules, Polymers & Chemistry in Everyday Life #### 1. Biomolecules - **Carbohydrates**: - **Monosaccharides**: Glucose, Fructose, Galactose. Reducing sugars. - **Disaccharides**: Sucrose (non-reducing), Maltose (reducing), Lactose (reducing). - **Polysaccharides**: Starch, Cellulose, Glycogen. - **Glucose**: Aldohexose, cyclic hemiacetal forms ($\alpha$-D-Glucose, $\beta$-D-Glucose). Mutarotation. - **Fructose**: Ketohexose, cyclic hemiketal forms. - **Proteins**: - **Amino Acids**: Building blocks. Zwitterionic form. Peptide bond formation. - **Essential Amino Acids**: Cannot be synthesized by body. - **Structure**: Primary (sequence), Secondary (helix, sheet), Tertiary (3D folding), Quaternary (multiple subunits). - **Denaturation**: Loss of biological activity due to change in 2°, 3°, 4° structure (heat, pH change). - **Vitamins**: Organic compounds required in small amounts for growth and metabolism. - **Fat-soluble**: A, D, E, K. - **Water-soluble**: B-complex, C. - **Nucleic Acids**: DNA, RNA. - **Components**: Pentose sugar (deoxyribose/ribose), Phosphate group, Nitrogenous bases (A, T, C, G, U). - **DNA Structure**: Double helix, A-T, G-C base pairing. - **Hormones**: Chemical messengers. (e.g., Insulin, Adrenaline). #### 2. Polymers - **Classification**: - **Natural**: Starch, Cellulose, Proteins, Natural Rubber. - **Synthetic**: Polyethylene, PVC, Nylon, Bakelite, Terylene. - **Types of Polymerization**: - **Addition Polymerization**: Monomers add without elimination of small molecules (e.g., Alkene polymerization, Polythene, PVC, Teflon, Polyacrylonitrile). - **Condensation Polymerization**: Monomers combine with elimination of small molecules (e.g., $H_2O, NH_3$) (e.g., Nylon 6,6, Terylene, Bakelite). - **Examples**: - **Polythene**: Ethene. Low density (LDPE), High density (HDPE). - **PVC**: Vinyl chloride. - **Teflon**: Tetrafluoroethene. - **Nylon 6,6**: Hexamethylenediamine + Adipic acid. - **Nylon 6**: Caprolactam. - **Terylene (Dacron)**: Ethylene glycol + Terephthalic acid. - **Bakelite**: Phenol + Formaldehyde. Thermosetting plastic. - **Natural Rubber**: Isoprene (cis-1,4-polyisoprene). Vulcanization with Sulfur. - **Biodegradable Polymers**: PHBV, Nylon 2-Nylon 6. #### 3. Chemistry in Everyday Life - **Drugs**: - **Analgesics**: Pain relievers (e.g., Aspirin, Paracetamol). - **Antipyretics**: Reduce fever (e.g., Aspirin, Paracetamol). - **Antiseptics**: Applied to living tissues (e.g., Dettol, Savlon). - **Disinfectants**: Applied to inanimate objects (e.g., Phenol, Chlorine). - **Antibiotics**: Treat bacterial infections (e.g., Penicillin, Chloramphenicol). - **Antacids**: Neutralize excess stomach acid (e.g., $Mg(OH)_2, Al(OH)_3$). - **Antihistamines**: Counter allergies (e.g., Seldane, Terfenadine). - **Tranquilizers**: Reduce stress/anxiety (e.g., Equanil, Barbiturates). - **Food Preservatives**: Prevent spoilage (e.g., Sodium benzoate, Salt, Sugar). - **Artificial Sweeteners**: Saccharin, Aspartame, Sucralose, Alitame. - **Soaps & Detergents**: - **Soaps**: Sodium/Potassium salts of long chain fatty acids. - **Detergents**: Synthetic, work in hard water. Anionic, Cationic, Non-ionic.