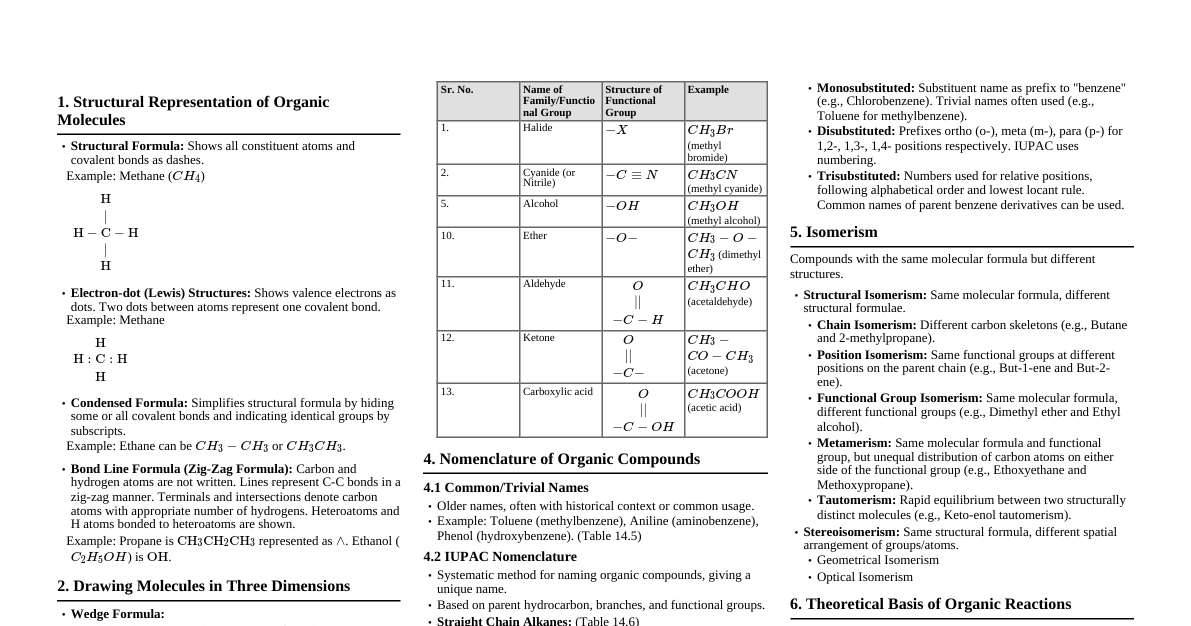
1. General Introduction to Organic Chemistry Organic compounds contain carbon, forming covalent bonds with other C atoms and elements like H, O, N, S, P, halogens. Initially, 'vital force' theory (Berzelius) for organic compound formation was disproved by Wöhler (urea synthesis from ammonium cyanate, 1828), Kolbe (acetic acid, 1845), and Berthelot (methane, 1856). Key applications: life sustenance (DNA, proteins), materials (clothing, fuels, polymers, dyes, medicines). 2. Tetravalence of Carbon & Molecular Shapes Carbon is tetravalent, forming covalent bonds. Hybridization: Explains shapes and bond properties. $sp^3$: Methane ($CH_4$), tetrahedral, 109.5° bond angle. $sp^2$: Ethene ($C_2H_4$), trigonal planar, 120° bond angle. $sp$: Ethyne ($C_2H_2$), linear, 180° bond angle. Bond Length & Strength: Influenced by hybridization. Higher s-character $\implies$ shorter, stronger bonds, greater electronegativity. $sp > sp^2 > sp^3$ in terms of s-character, electronegativity, bond strength, and inverse bond length. $\pi$ (Pi) Bonds: Formed by parallel overlap of p-orbitals. Electron cloud above/below bonding plane. Restricted rotation around C=C bonds. Provide reactive centers due to easily available electrons. 3. Representation of Organic Compounds Complete Structural Formulas: Shows all atoms and bonds (dashes). Single bond: $-$ Double bond: $=$ Triple bond: $\equiv$ Lone pairs may or may not be shown. Condensed Structural Formulas: Omits some/all dashes, indicates identical groups with subscripts. Example: Ethane ($CH_3CH_3$), Ethene ($H_2C=CH_2$), Ethyne ($HC \equiv CH$), Methanol ($CH_3OH$). Bond-line Structural Formulas (Zig-zag): C and H atoms not shown (implied at vertices/ends). Lines represent C-C bonds. Heteroatoms (O, Cl, N, etc.) are explicitly written. Terminals denote $-CH_3$ groups (unless functional group present). Line junctions denote carbon atoms with appropriate number of H to satisfy valency. Cyclic compounds: represented by polygons (e.g., cyclopropane, cyclopentane, chlorocyclohexane). Three-Dimensional (3-D) Representation: Solid wedge ($\blacktriangle$): Bond projecting out of plane, towards observer. Dashed wedge ($\dots$): Bond projecting out of plane, away from observer. Normal line ($-$): Bond in the plane of the paper. Example: Methane ($CH_4$) Molecular Models: Framework, Ball-and-stick, Space-filling models for visualization. 4. Classification of Organic Compounds Acyclic (Open Chain) / Aliphatic Compounds: Straight or branched chains (e.g., ethane, isobutane, acetaldehyde, acetic acid). Cyclic (Closed Chain) / Ring Compounds: Alicyclic Compounds: Carbon atoms form a ring, properties similar to aliphatic (e.g., cyclopropane, cyclohexane, cyclohexene). Aromatic Compounds: Special types, includes benzenoid (benzene, aniline, naphthalene) and non-benzenoid (tropone). Heterocyclic Compounds: Ring contains atoms other than carbon (e.g., tetrahydrofuran, furan, thiophene, pyridine). Functional Groups: Atom/group responsible for characteristic chemical properties (e.g., $-\text{OH}$, $-\text{CHO}$, $-\text{COOH}$). Homologous Series: Series of compounds with same functional group, differing by a $-\text{CH}_2$ unit (e.g., alkanes, alkenes, alcohols). Polyfunctional Compounds: Contain two or more identical or different functional groups. 5. IUPAC Nomenclature Common/Trivial Names: Based on origin or properties (e.g., formic acid, urea, chloroform). Systematic Naming (IUPAC): Identify parent hydrocarbon and functional groups. Alkanes: Straight Chain: Suffix "-ane", prefix indicates carbon count (e.g., methane, ethane). Branched Chain: Longest carbon chain identified as parent. Number chain to give substituents lowest possible numbers. Alkyl groups (derived by removing H from alkane) are prefixed (e.g., methyl, ethyl, propyl, butyl). Different alkyl groups listed alphabetically. Identical substituents: use di-, tri-, tetra- prefixes. Branched alkyl groups: carbon attached to parent is numbered 1. Cyclic Compounds: Prefix "cyclo-" to alkane name. Number substituents on ring. Compounds with Functional Groups: Identify principal functional group (determines suffix). Longest carbon chain containing functional group is numbered to give functional group lowest number. Order of preference for functional groups (decreasing priority): $-\text{COOH} > -\text{SO}_3\text{H} > -\text{COOR} > -\text{COCl} > -\text{CONH}_2 > -\text{CN} > -\text{HC=O} > \text{>C=O} > -\text{OH} > -\text{NH}_2 > \text{>C=C \text{-C}\equiv\text{C-}$. Prefix substituents: $-\text{R}$, $-\text{CH}_x$, halogens ($\text{F, Cl, Br, I}$), $-\text{NO}_2$, alkoxy $(-\text{OR})$. If multiple functional groups of same type, use di-, tri-, etc. before suffix. Substituted Benzene Compounds: Substituent as prefix to "benzene" (e.g., methylbenzene, nitrobenzene). Disubstituted: Number ring carbons to give lowest numbers (e.g., 1,3-dibromobenzene). Trivial: ortho (1,2), meta (1,3), para (1,4). Tri/higher substituted: Use lowest locant rule, alphabetical order. Phenyl ($C_6H_5-$) as substituent when benzene ring is attached to an alkane with a functional group. 6. Isomerism Compounds with same molecular formula but different properties. Structural Isomerism: Same molecular formula, different connectivity of atoms. Chain Isomerism: Different carbon skeletons (e.g., pentane, isopentane, neopentane for $C_5H_{12}$). Position Isomerism: Different position of substituent/functional group on carbon skeleton (e.g., propan-1-ol, propan-2-ol for $C_3H_8O$). Functional Group Isomerism: Different functional groups (e.g., aldehyde and ketone for $C_3H_6O$). Metamerism: Different alkyl chains on either side of functional group (e.g., methoxypropane and ethoxyethane for $C_4H_{10}O$). Stereoisomerism: Same constitution, different spatial arrangement of atoms. Geometrical Isomerism Optical Isomerism 7. Organic Reaction Mechanism Covalent Bond Fission: Heterolytic Cleavage: Bond breaks, shared pair of electrons stays with one fragment. Forms: Carbocation ($C^+$) (sextet, positive charge, $sp^2$ hybridized, trigonal planar) and Carbanion ($C^-$) (valence octet, lone pair, negative charge, $sp^3$ hybridized, distorted tetrahedral). Reactions: Ionic, heteropolar, polar. Homolytic Cleavage: Each bonded atom gets one electron from shared pair. Forms: Free Radicals (neutral, unpaired electron, reactive). Reactions: Free radical, homopolar, nonpolar. Substrate and Reagent: Substrate: Molecule contributing carbon for new bond formation. Reagent: Attacking species. Nucleophile (Nu:): Electron-rich species, donates electron pair (e.g., $HO^-$, $CN^-$, $R_3N$). Attacks electrophilic centers. Electrophile (E$^+$): Electron-deficient species, accepts electron pair (e.g., carbocations, carbonyl groups, alkyl halides). Attacks nucleophilic centers. Electron Movement (Curved Arrows): Full-headed arrow ($\curvearrowright$): Movement of an electron pair. Half-headed (fish hook) arrow ($\curvearrowright$): Movement of a single electron. Electronic Displacements in Covalent Bonds (Permanent): Inductive Effect (I-effect): Polarity in $\sigma$-bond due to electronegativity difference, transmitted through chain, diminishes with distance. Electron-withdrawing (-I): Halogens, $-\text{NO}_2$, $-\text{CN}$, $-\text{COOH}$. Electron-donating (+I): Alkyl groups ($-\text{CH}_3$, $-\text{CH}_2\text{CH}_3$). Resonance Effect (R-effect) / Mesomeric Effect (M-effect): Polarity produced by interaction of $\pi$-bonds or between $\pi$-bond and lone pair on adjacent atom. Resonance Structures: Hypothetical, contribute to actual hybrid. Same nuclei positions, same number of unpaired electrons. More covalent bonds, complete octets, less charge separation, negative charge on electronegative atom, positive on electropositive atom $\implies$ more stable. Positive Resonance Effect (+R): Electron transfer away from atom/group to conjugated system (e.g., $-\text{OH}$, $-\text{NH}_2$, halogens). Negative Resonance Effect (-R): Electron transfer towards atom/group from conjugated system (e.g., $-\text{NO}_2$, $-\text{CHO}$, $-\text{COOH}$). Conjugated system: Alternate single and double bonds. Resonance stabilization energy: Energy difference between actual structure and lowest energy resonance structure. Hyperconjugation: Delocalization of $\sigma$-electrons of C-H bond of an alkyl group into adjacent unsaturated system or p-orbital. Permanent effect, also called "no bond resonance". Stabilizes carbocations and alkenes. More alkyl groups $\implies$ greater hyperconjugation $\implies$ greater stability of carbocation. Stability order: Tertiary $>$ Secondary $>$ Primary $>$ Methyl. Electronic Displacements (Temporary): Electromeric Effect (E-effect): Complete transfer of $\pi$-electrons to one atom of a multiple bond in presence of attacking reagent. Positive (+E): $\pi$-electrons transfer to atom where reagent attaches. Negative (-E): $\pi$-electrons transfer away from atom where reagent attaches. Predominates over inductive effect. Types of Organic Reactions: Substitution, Addition, Elimination, Rearrangement. 8. Named Reactions Wöhler Synthesis: $NH_4CNO \xrightarrow{\text{heat}} (NH_2)_2CO$ (urea). First synthesis of an organic compound from inorganic precursors. Liebig's Method (for C & H Analysis): Combustion of organic compound with $CuO$. Dumas Method (for Nitrogen Analysis): Combustion with $CuO$ to produce elemental $N_2$. Kjeldahl's Method (for Nitrogen Analysis): Digestion with $H_2SO_4$ to $(NH_4)_2SO_4$, followed by distillation of $NH_3$ and titration. Carius Method (for Halogen/Sulphur/Phosphorus Analysis): Heating with fuming $HNO_3$ in a sealed tube to convert elements to inorganic compounds for gravimetric analysis. Lassaigne's Test: Fusion with sodium metal to convert covalently bonded N, S, halogens into ionic compounds for qualitative detection. (Note: This section will be expanded as more specific named reactions are introduced in later chapters, e.g., Wurtz, Friedel-Crafts, Aldol, Cannizzaro, etc.) 9. Methods of Purification of Organic Compounds Sublimation: Separates sublimable solids from non-sublimable impurities (solid to vapor without liquid phase). Crystallization: Based on solubility difference in a solvent at different temperatures. Impure compound dissolved in hot solvent, then cooled to crystallize pure compound. Impurities removed by filtration. Activated charcoal removes color impurities. Distillation: Separates liquids based on boiling point differences. Simple Distillation: For liquids with significant boiling point difference. Fractional Distillation: For liquids with small boiling point difference, uses fractionating column. Distillation under Reduced Pressure: For high-boiling or decomposable liquids, reduces pressure to lower boiling point. Steam Distillation: For steam-volatile and water-immiscible compounds. Differential Extraction: Separates organic compound from aqueous medium using an immiscible organic solvent where compound is more soluble. Chromatography: Separates mixtures based on differential movement through stationary and mobile phases. Adsorption Chromatography: Different adsorption on adsorbent. Column Chromatography: Mixture applied to adsorbent column, eluant flows, components separate. Thin Layer Chromatography (TLC): Mixture spotted on adsorbent-coated plate, eluant moves up, components separate. $R_f$ value = (distance substance moves) / (distance solvent moves). Partition Chromatography: Differential partitioning between stationary (water in paper) and mobile phase. Paper Chromatography: Mixture spotted on paper, solvent moves up, components separate. Purity Check: Melting/boiling points (sharp for pure compounds). Modern: CHN elemental analyzer. 10. Qualitative Analysis of Organic Compounds Detection of Carbon & Hydrogen: Heat with $CuO$. Carbon $\to CO_2$ (turbidity with lime-water, $Ca(OH)_2$). Hydrogen $\to H_2O$ (turns anhydrous $CuSO_4$ blue). Lassaigne's Test (for N, S, Halogens): Fuse organic compound with Na metal to convert covalent elements to ionic. Extract with water (Sodium Fusion Extract). Nitrogen: Extract + $FeSO_4$ + conc. $H_2SO_4 \to$ Prussian blue ($Fe_4[Fe(CN)_6]_3$). Sulphur: Extract + acetic acid + lead acetate $\to$ black precipitate ($PbS$). Extract + sodium nitroprusside $\to$ violet color. If N & S both present $\to$ NaSCN $\to$ blood red with $Fe^{3+}$ ($[Fe(SCN)]^{2+}$). Halogens (Cl, Br, I): Extract + $HNO_3$ (to remove $CN^-, S^{2-}$ interference) + $AgNO_3 \to AgX$ precipitate. $AgCl$: white, soluble in $NH_4OH$. $AgBr$: yellowish, sparingly soluble in $NH_4OH$. $AgI$: yellow, insoluble in $NH_4OH$. Phosphorus: Heat with oxidizing agent (Na peroxide) $\to$ phosphate. Extract + $HNO_3$ + ammonium molybdate $\to$ yellow color/precipitate (ammonium phosphomolybdate). 11. Quantitative Analysis Determines mass percent of elements for empirical/molecular formula. Carbon & Hydrogen (Liebig's Method): Burn known mass of compound in $O_2$/$CuO$. $H_2O$ absorbed by anhydrous $CaCl_2$. $CO_2$ absorbed by $KOH$ solution. Calculate % C and % H from masses of $H_2O$ and $CO_2$. Nitrogen: Dumas Method: Heat compound with $CuO$ in $CO_2$ atmosphere $\to$ free $N_2$. Collect $N_2$ over $KOH$ solution (absorbs $CO_2$). Calculate % N from volume of $N_2$ at STP. Kjeldahl's Method: Heat compound with conc. $H_2SO_4 \to (NH_4)_2SO_4$. Treat with $NaOH \to NH_3$ gas. Absorb $NH_3$ in excess standard $H_2SO_4$. Titrate unreacted $H_2SO_4$ with standard alkali. Calculate % N from amount of $NH_3$ produced. Not applicable for nitro, azo groups, or N in ring (e.g., pyridine). Halogens (Carius Method): Heat compound with fuming $HNO_3$/$AgNO_3$ in Carius tube $\to AgX$ precipitate. Filter, wash, dry, weigh $AgX$. Calculate % Halogen from mass of $AgX$. Sulphur (Carius Method): Heat compound with $Na_2O_2$/fuming $HNO_3$ in Carius tube $\to H_2SO_4$. Precipitate as $BaSO_4$ with $BaCl_2$. Filter, wash, dry, weigh $BaSO_4$. Calculate % S from mass of $BaSO_4$. Phosphorus: Oxidize to phosphoric acid. Precipitate as ammonium phosphomolybdate or $Mg_2P_2O_7$. Weigh precipitate. Calculate % P. Oxygen: Usually calculated by difference (100% - sum of other elements). Direct method: Decompose in $N_2$ stream, pass over red-hot coke $\to CO$. Pass $CO$ over $I_2O_5 \to CO_2 + I_2$. Calculate % O from $CO_2$ or $I_2$.