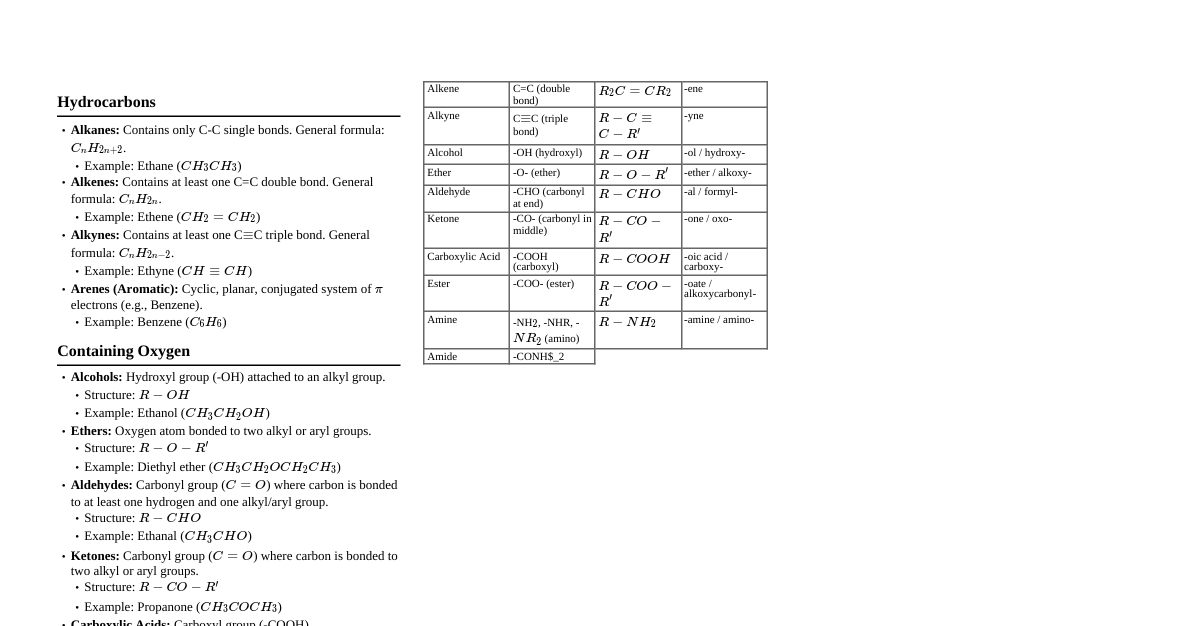
1. Basic Concepts Organic Chemistry: Study of carbon-containing compounds. Carbon forms 4 bonds. Hybridization: $sp^3$: 4 single bonds, tetrahedral, $\approx 109.5^\circ$ bond angles (e.g., alkanes) $sp^2$: 1 double bond, 2 single bonds, trigonal planar, $\approx 120^\circ$ (e.g., alkenes, carbonyls) $sp$: 1 triple bond, 1 single bond; or 2 double bonds, linear, $180^\circ$ (e.g., alkynes, nitriles) Functional Groups: Specific groups of atoms responsible for characteristic chemical reactions. Isomers: Compounds with the same molecular formula but different structures. Constitutional Isomers: Different connectivity. Stereoisomers: Same connectivity, different arrangement in space. Enantiomers: Non-superimposable mirror images (chiral center). Diastereomers: Stereoisomers that are not mirror images (e.g., cis/trans, multiple chiral centers). 2. Alkanes, Alkenes, Alkynes Alkanes (C-C single bonds) General Formula: $C_nH_{2n+2}$ Saturated hydrocarbons, relatively unreactive. Reactions: Radical halogenation (e.g., $CH_4 + Cl_2 \xrightarrow{hv} CH_3Cl + HCl$) Nomenclature: -ane suffix. Alkenes (C=C double bonds) General Formula: $C_nH_{2n}$ Unsaturated hydrocarbons, reactive due to $\pi$ bond. Reactions: Electrophilic addition (Markovnikov's rule for unsymmetrical alkenes). Hydrogenation: $C=C + H_2 \xrightarrow{Pd/C} C-C$ Halogenation: $C=C + Br_2 \rightarrow$ vicinal dibromide Hydrohalogenation: $C=C + HBr \rightarrow$ alkyl halide Hydration: $C=C + H_2O \xrightarrow{H^+} \rightarrow$ alcohol Nomenclature: -ene suffix, specify stereochemistry (cis/trans or E/Z). Alkynes (C$\equiv$C triple bonds) General Formula: $C_nH_{2n-2}$ Unsaturated hydrocarbons, reactive due to two $\pi$ bonds. Terminal alkynes are acidic. Reactions: Electrophilic addition (similar to alkenes, but can add twice). Hydrogenation: $C\equiv C + H_2 \xrightarrow{Pd/C} C=C$; $C\equiv C + 2H_2 \xrightarrow{Pd/C} C-C$ Partial Hydrogenation: $C\equiv C + H_2 \xrightarrow{Lindlar's Cat} cis-alkene$ Partial Hydrogenation: $C\equiv C + Na/NH_3(l) \rightarrow trans-alkene$ Nomenclature: -yne suffix. 3. Aromatic Compounds Aromaticity (Hückel's Rule): Cyclic Planar Fully conjugated (continuous overlap of p-orbitals) $(4n+2)\pi$ electrons ($n=0, 1, 2, ...$) Benzene Reactions: Electrophilic Aromatic Substitution (EAS) Halogenation: $\text{Benzene} + Br_2 \xrightarrow{FeBr_3} \text{Bromobenzene}$ Nitration: $\text{Benzene} + HNO_3 \xrightarrow{H_2SO_4} \text{Nitrobenzene}$ Sulfonation: $\text{Benzene} + H_2SO_4 \xrightarrow{SO_3} \text{Benzenesulfonic Acid}$ Friedel-Crafts Alkylation: $\text{Benzene} + R-Cl \xrightarrow{AlCl_3} \text{Alkylbenzene}$ Friedel-Crafts Acylation: $\text{Benzene} + R-CO-Cl \xrightarrow{AlCl_3} \text{Acylbenzene}$ Substituent Effects in EAS: Activating & Ortho/Para Directors: Alkyl groups, $-OH, -OR, -NH_2, -NR_2$ Deactivating & Meta Directors: $-NO_2, -SO_3H, -CN, -COOH, -COOR, -CHO, -COR$ Deactivating & Ortho/Para Directors: Halogens ($F, Cl, Br, I$) 4. Alcohols, Ethers, Amines Alcohols (R-OH) Nomenclature: -ol suffix. Classified as primary ($1^\circ$), secondary ($2^\circ$), or tertiary ($3^\circ$). Reactions: Oxidation: $1^\circ \text{ alcohol} \xrightarrow{PCC} \text{aldehyde} \xrightarrow{CrO_3/H_2SO_4} \text{carboxylic acid}$ Oxidation: $2^\circ \text{ alcohol} \xrightarrow{CrO_3/H_2SO_4} \text{ketone}$ $3^\circ \text{ alcohols}$ are not easily oxidized. Dehydration: Alcohol $\xrightarrow{H_2SO_4, \Delta} \text{Alkene}$ (E1/E2) Substitution: Alcohol $\xrightarrow{HBr \text{ or } HCl} \text{Alkyl Halide}$ ($S_N1/S_N2$) Ethers (R-O-R') Nomenclature: Alkoxyalkane. Relatively unreactive, good solvents. Synthesis: Williamson Ether Synthesis ($R-O^-Na^+ + R'-X \rightarrow R-O-R'$). Amines (R-$NH_2$, R-$NHR'$, R-$NR'_2$) Nomenclature: -amine suffix or N-substituted alkylamines. Classified as $1^\circ, 2^\circ, 3^\circ$. Basic: Lone pair on Nitrogen. React with acids to form ammonium salts. Reactions: Nucleophilic substitution, acylation. 5. Carbonyl Compounds Aldehydes (R-CHO) & Ketones (R-CO-R') Nomenclature: Aldehydes (-al), Ketones (-one). Reactions (Nucleophilic Addition to Carbonyl): Addition of $HCN \rightarrow$ Cyanohydrin Addition of Grignard Reagent ($R-MgX$) $\rightarrow$ Alcohol Reduction with $NaBH_4$ or $LiAlH_4 \rightarrow$ Alcohol Formation of Imines ($R-CHO/R_2C=O + R'-NH_2 \rightarrow R-CH=NR'/R_2C=NR'$) Wittig Reaction: Aldehyde/Ketone + Ylide $\rightarrow$ Alkene Aldehydes are more reactive than ketones towards nucleophiles. Alpha-Hydrogen Acidity: $\alpha$-hydrogens are acidic due to resonance stabilization of enolate. Enol/Keto Tautomerism: $R_2CH-CHO \rightleftharpoons R_2C=CH-OH$ Carboxylic Acids (R-COOH) Nomenclature: -oic acid suffix. Acidic: Lone pair on Oxygen, resonance stabilized carboxylate anion. Reactions (Nucleophilic Acyl Substitution): Esterification: Carboxylic acid + Alcohol $\xrightarrow{H^+} \text{Ester}$ ($R-COOR'$) Amide Formation: Carboxylic acid + Amine $\xrightarrow{\Delta} \text{Amide}$ ($R-CONR'_2$) Reduction with $LiAlH_4 \rightarrow$ Primary Alcohol Carboxylic Acid Derivatives Esters (R-COOR'): Sweet-smelling. Hydrolysis to carboxylic acid and alcohol. Amides (R-CONR'_2): Most stable derivative. Hydrolysis requires strong acid/base. Acid Chlorides (R-COCl): Most reactive. React with water, alcohols, amines. Acid Anhydrides (R-CO-O-CO-R'): Reactive. React with water, alcohols, amines. 6. Reaction Mechanisms Overview $S_N1$ (Substitution Nucleophilic Unimolecular): Rate: $k[RX]$ Favored by $3^\circ$ alkyl halides, weak nucleophiles, polar protic solvents. Carbocation intermediate, racemization of stereocenter. $S_N2$ (Substitution Nucleophilic Bimolecular): Rate: $k[RX][Nu^-]$ Favored by $1^\circ$ alkyl halides, strong nucleophiles, polar aprotic solvents. Concerted reaction, inversion of configuration (Walden inversion). $E1$ (Elimination Unimolecular): Rate: $k[RX]$ Favored by $3^\circ$ alkyl halides, weak bases, polar protic solvents. Carbocation intermediate, Zaitsev's rule (most substituted alkene). $E2$ (Elimination Bimolecular): Rate: $k[RX][Base]$ Favored by $1^\circ, 2^\circ, 3^\circ$ alkyl halides, strong bases, polar aprotic solvents. Concerted reaction, anti-periplanar geometry required. Zaitsev's rule. 7. Spectroscopy NMR (Nuclear Magnetic Resonance) $^{1}$H NMR: Chemical Shift ($\delta$): Position of signal, indicates electronic environment. Alkyl protons: $0.9-1.5$ ppm Protons next to electronegative atom (e.g., C-O-H): $3.5-4.5$ ppm Allylic protons: $1.7-2.3$ ppm Vinylic protons: $4.5-6.0$ ppm Aromatic protons: $6.5-8.0$ ppm Aldehyde protons: $9.0-10.0$ ppm Carboxylic acid protons: $10.0-12.0$ ppm Integration: Area under signal, proportional to number of equivalent protons. Multiplicity (Splitting): $(n+1)$ rule, where $n$ is number of equivalent adjacent protons. $\text{Singlet (s):}$ 0 neighbors $\text{Doublet (d):}$ 1 neighbor $\text{Triplet (t):}$ 2 neighbors $\text{Quartet (q):}$ 3 neighbors $^{13}$C NMR: Shows number of unique carbon environments. Broad range of chemical shifts ($0-220$ ppm). Does not typically show splitting (proton decoupled). IR (Infrared Spectroscopy) Identifies functional groups based on bond vibrations. Key Absorptions: $O-H$ (alcohol): $3200-3600 \text{ cm}^{-1}$ (broad) $O-H$ (carboxylic acid): $2500-3300 \text{ cm}^{-1}$ (very broad) $N-H$ (amine): $3300-3500 \text{ cm}^{-1}$ (1 or 2 sharp peaks) $C-H$ (alkane): $2850-2960 \text{ cm}^{-1}$ (sharp) $C=O$ (carbonyl): $1650-1780 \text{ cm}^{-1}$ (strong, sharp) $C=C$ (alkene): $1620-1680 \text{ cm}^{-1}$ (medium) $C\equiv C$ (alkyne): $2100-2260 \text{ cm}^{-1}$ (weak) $C\equiv N$ (nitrile): $2200-2260 \text{ cm}^{-1}$ (medium) Mass Spectrometry (MS) Determines molecular weight and fragmentation pattern. Molecular Ion ($M^+$): Mass corresponding to the molecular weight of the compound. Base Peak: Most abundant ion (tallest peak). Isotopes: $M+1$ peak (due to $^{13}C$), $M+2$ peak (e.g., $Cl_2$ or $Br_2$).