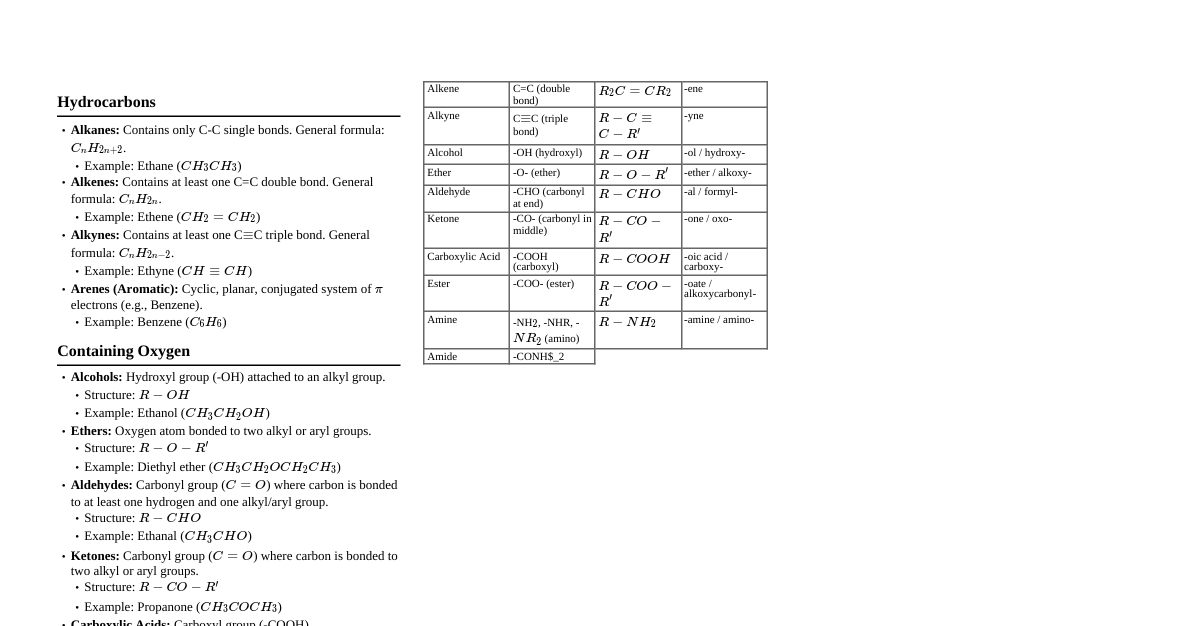
### Introduction to GOC - **Definition:** General Organic Chemistry (GOC) deals with the fundamental principles governing organic compounds, including their structure, bonding, reactivity, and reaction mechanisms. - **Organic Compounds:** Compounds containing carbon, usually bonded to hydrogen, and often with oxygen, nitrogen, sulfur, or halogens. - **Key Elements:** C, H, O, N, S, P, Halogens (F, Cl, Br, I). ### Hybridization and Bonding - **Carbon's Valency:** Carbon is tetravalent, forming four bonds. - **Types of Hybridization:** - $sp^3$: Single bonds (e.g., alkanes). Tetrahedral geometry, 109.5° bond angle. - $sp^2$: Double bonds (e.g., alkenes). Trigonal planar geometry, 120° bond angle. - $sp$: Triple bonds (e.g., alkynes) or two double bonds (e.g., allenes). Linear geometry, 180° bond angle. - **Bond Length & Strength:** - Single bond > Double bond > Triple bond (length) - Triple bond > Double bond > Single bond (strength) - **Sigma ($\sigma$) Bonds:** Formed by head-on overlap of atomic orbitals. Stronger. - **Pi ($\pi$) Bonds:** Formed by lateral overlap of p-orbitals. Weaker, responsible for reactivity in multiple bonds. ### Electronic Effects: Inductive Effect - **Definition:** Permanent displacement of $\sigma$-electrons along a carbon chain due to difference in electronegativity between atoms. - **Types:** - **+I Effect (Electron-donating):** Alkyl groups ($-\text{CH}_3$, $-\text{CH}_2\text{CH}_3$, etc.), $-\text{COO}^-$. Increases electron density. - **-I Effect (Electron-withdrawing):** $-\text{NO}_2$, $-\text{CN}$, $-\text{COOH}$, Halogens $(-\text{F} > -\text{Cl} > -\text{Br} > -\text{I})$. Decreases electron density. - **Effect on Acidity/Basicity:** - **Acidity:** Increased by -I effect (stabilizes conjugate base), decreased by +I effect. - **Basicity:** Increased by +I effect (stabilizes conjugate acid), decreased by -I effect. - **Distance Dependence:** Decreases rapidly with distance from the electronegative atom. ### Electronic Effects: Resonance (Mesomeric Effect) - **Definition:** Delocalization of $\pi$-electrons or lone pair electrons within a conjugated system. Represented by resonance structures. - **Conditions for Resonance:** Presence of conjugated system (alternating single and multiple bonds, or multiple bond adjacent to atom with lone pair/empty orbital). - **Types:** - **+R/+M Effect (Electron-donating):** Groups with lone pairs ($-\text{OH}$, $-\text{OR}$, $-\text{NH}_2$, Halogens). Donates electrons to the conjugated system. - **-R/-M Effect (Electron-withdrawing):** Groups with multiple bonds where the atom directly attached to the system is more electronegative ($-\text{NO}_2$, $-\text{CN}$, $-\text{CHO}$, $-\text{COOH}$). Withdraws electrons from the conjugated system. - **Resonance Hybrid:** The true structure of a molecule exhibiting resonance, which is an average of all contributing resonance structures. It is more stable than any single resonance structure. - **Rules for Resonance Structures:** - Only electrons move, not atoms. - Total number of electrons and formal charges must be conserved. - Major contributors are those with more covalent bonds, fewer charge separations, and negative charge on more electronegative atom. ### Electronic Effects: Hyperconjugation - **Definition:** Delocalization of $\sigma$-electrons of a C-H bond with an adjacent empty p-orbital (carbocation), $\pi$-bond (alkene), or p-orbital containing an unpaired electron (free radical). Also known as "no-bond resonance." - **Conditions:** Presence of $\alpha$-hydrogens (hydrogens on carbon adjacent to the unsaturated system or carbocation). - **Stability Order:** - **Carbocations:** $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ (due to hyperconjugation and inductive effect). - **Alkenes:** More substituted alkenes are more stable (more $\alpha$-hydrogens). - **Free Radicals:** $3^\circ > 2^\circ > 1^\circ > \text{methyl}$. - **Effect:** Stabilizes carbocations, free radicals, and alkenes by spreading out electron density. ### Acidity and Basicity - **Acids (Brønsted-Lowry):** Proton ($H^+$) donors. - **Bases (Brønsted-Lowry):** Proton ($H^+$) acceptors. - **Factors Affecting Acidity:** 1. **Electronegativity:** Acidity increases across a period (e.g., $\text{CH}_4 sp^2 > sp^3$ (e.g., alkynes are more acidic than alkenes/alkanes). 4. **Inductive Effect:** Electron-withdrawing groups (-I) increase acidity; electron-donating groups (+I) decrease acidity. 5. **Resonance Effect:** Resonance stabilization of the conjugate base increases acidity. - **Factors Affecting Basicity:** 1. **Availability of Lone Pair:** Greater availability of lone pair makes a compound more basic. 2. **Inductive Effect:** Electron-donating groups (+I) increase basicity; electron-withdrawing groups (-I) decrease basicity. 3. **Resonance Effect:** Delocalization of lone pair by resonance decreases basicity. 4. **Hybridization:** $sp^3 > sp^2 > sp$ (e.g., alkyl amines are more basic than imines/nitriles). ### Isomerism - **Definition:** Compounds with the same molecular formula but different structural or spatial arrangements of atoms. - **Structural Isomerism (Constitutional Isomerism):** Different connectivity of atoms. - **Chain Isomerism:** Different carbon skeleton arrangements (e.g., n-butane vs. isobutane). - **Position Isomerism:** Different position of functional group or substituent (e.g., 1-propanol vs. 2-propanol). - **Functional Group Isomerism:** Different functional groups (e.g., ethanol vs. dimethyl ether). - **Metamerism:** Different alkyl groups attached to the same functional group (e.g., diethyl ether vs. methyl propyl ether). - **Tautomerism:** Rapid equilibrium between two functional isomers, usually involving a proton shift and double bond shift (e.g., keto-enol tautomerism). - **Stereoisomerism:** Same connectivity but different spatial arrangement of atoms. - **Conformational Isomerism:** Can be interconverted by rotation around single bonds (e.g., ethane staggered/eclipsed, cyclohexane chair/boat). - **Configurational Isomerism:** Cannot be interconverted without breaking bonds. - **Geometrical Isomerism (cis-trans/E-Z):** Due to restricted rotation around a double bond or in cyclic compounds. - **cis/trans:** When two identical groups are on the same side (cis) or opposite sides (trans) of the double bond/ring. - **E/Z:** Used for more complex cases. E (entgegen - opposite) and Z (zusammen - together) based on Cahn-Ingold-Prelog priority rules. - **Optical Isomerism (Enantiomers/Diastereomers):** Due to presence of chiral center(s). - **Chiral Center:** A carbon atom bonded to four different groups. - **Enantiomers:** Non-superimposable mirror images. Have identical physical properties except for rotation of plane-polarized light (optical activity). - **Diastereomers:** Stereoisomers that are not mirror images. Different physical and chemical properties. - **Meso Compounds:** Possess chiral centers but are achiral due to an internal plane of symmetry. - **Racemic Mixture:** An equimolar mixture of enantiomers, optically inactive. ### Reaction Intermediates - **Definition:** Highly reactive, short-lived species formed during a reaction that are not present in the starting materials or final products. - **Carbocations:** - **Structure:** Carbon with a positive charge, $sp^2$ hybridized, trigonal planar geometry, empty p-orbital. - **Stability:** $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ (stabilized by +I and hyperconjugation). - **Reactions:** Electrophiles, readily accept electrons. Undergo rearrangements (hydride/alkyl shifts) to form more stable carbocations. - **Carbanions:** - **Structure:** Carbon with a negative charge and a lone pair, $sp^3$ hybridized, trigonal pyramidal geometry. - **Stability:** $\text{methyl} > 1^\circ > 2^\circ > 3^\circ$ (destabilized by +I effect). - **Reactions:** Nucleophiles, readily donate electrons. Strong bases. - **Free Radicals:** - **Structure:** Carbon with an unpaired electron, often $sp^2$ hybridized, trigonal planar or slightly pyramidal. - **Stability:** $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ (stabilized by hyperconjugation). - **Reactions:** Highly reactive, participate in radical reactions (e.g., halogenation of alkanes). - **Carbenes:** - **Structure:** Neutral, divalent carbon atom with two unshared electrons. Can be singlet (paired electrons, $sp^2$) or triplet (unpaired electrons, $sp$). - **Reactions:** Highly reactive electrophiles or nucleophiles. ### Nucleophiles and Electrophiles - **Nucleophiles (Nucleus-loving):** - **Definition:** Electron-rich species that donate an electron pair to form a new bond. - **Characteristics:** Possess lone pairs or $\pi$-electrons, often negatively charged or neutral with available electrons. - **Examples:** $\text{OH}^-$, $\text{Cl}^-$, $\text{NH}_3$, $\text{H}_2\text{O}$, alkenes, alkynes. - **Electrophiles (Electron-loving):** - **Definition:** Electron-deficient species that accept an electron pair to form a new bond. - **Characteristics:** Possess an empty orbital, often positively charged or neutral with an electron-deficient atom. - **Examples:** $\text{H}^+$, $\text{NO}_2^+$, $\text{BF}_3$, $\text{AlCl}_3$, carbocations, carbonyl carbons. ### Types of Organic Reactions - **Substitution Reactions:** An atom or group is replaced by another atom or group. - **Nucleophilic Substitution (SN1, SN2):** Common for alkyl halides. - **Electrophilic Aromatic Substitution (EAS):** Common for benzene and its derivatives. - **Free Radical Substitution:** For alkanes (e.g., halogenation). - **Addition Reactions:** Atoms or groups are added across a multiple bond (double or triple bond). - **Electrophilic Addition:** Common for alkenes and alkynes (e.g., HBr addition). - **Nucleophilic Addition:** Common for carbonyl compounds (aldehydes, ketones). - **Free Radical Addition:** Less common, but occurs (e.g., HBr addition in presence of peroxides). - **Elimination Reactions:** Atoms or groups are removed from a molecule to form a multiple bond. - **E1, E2 Reactions:** Common for alkyl halides and alcohols. - **Saytzeff's Rule:** In elimination, the major product is the more substituted alkene (more stable). - **Rearrangement Reactions:** A change in the connectivity of atoms within a molecule, often leading to a more stable structure (e.g., carbocation rearrangements). - **Redox Reactions:** Oxidation and reduction in organic compounds. - **Oxidation:** Increase in C-O bonds, decrease in C-H bonds (e.g., alcohol to aldehyde/ketone to carboxylic acid). - **Reduction:** Decrease in C-O bonds, increase in C-H bonds (e.g., aldehyde/ketone to alcohol). ### Stereochemistry in Reactions - **SN1 Reactions:** Proceed via a carbocation intermediate, leading to racemization if the carbocation is chiral (product is a mixture of enantiomers). - **SN2 Reactions:** Involve backside attack, leading to inversion of configuration at the chiral center (Walden inversion). - **Electrophilic Addition to Alkenes:** - **Syn-addition:** Both added groups on the same face of the alkene (e.g., catalytic hydrogenation). - **Anti-addition:** Added groups on opposite faces (e.g., bromination). - **E2 Reactions:** Typically proceed via an anti-periplanar transition state, leading to specific stereochemical outcomes.