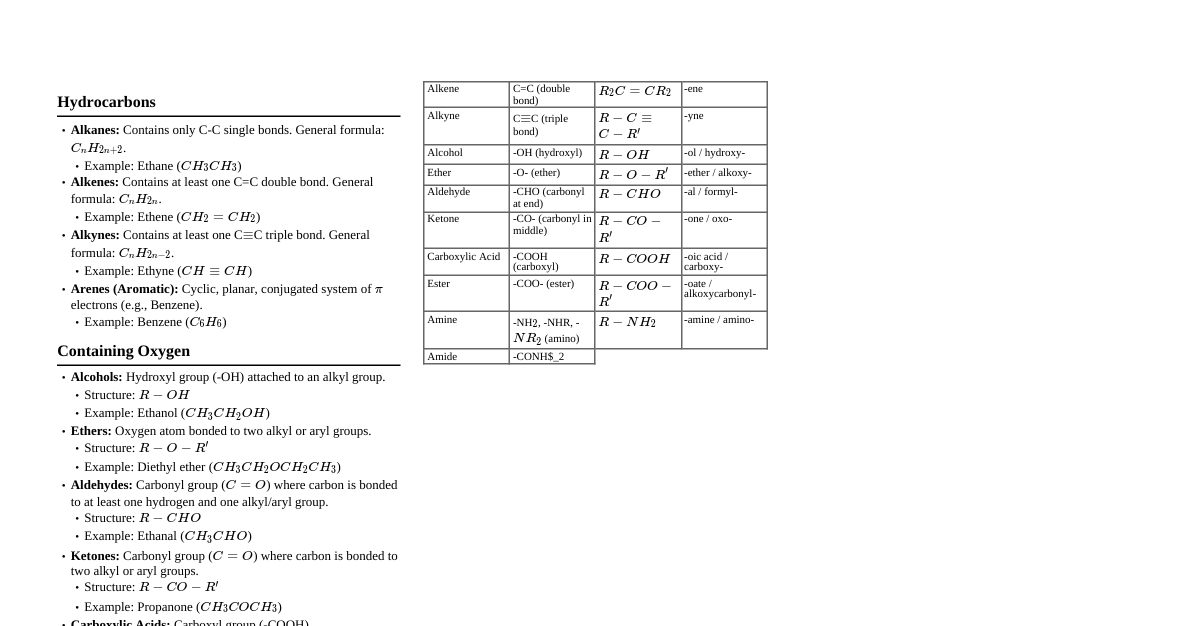
1. Hybridization & Structure Hybridization: Mixing of atomic orbitals to form new hybrid orbitals. $sp^3$: 4 sigma bonds, tetrahedral, 109.5° (e.g., alkanes) $sp^2$: 3 sigma bonds, 1 pi bond, trigonal planar, 120° (e.g., alkenes) $sp$: 2 sigma bonds, 2 pi bonds, linear, 180° (e.g., alkynes) Steric Number = (Number of sigma bonds) + (Number of lone pairs). 2 $\rightarrow sp$ 3 $\rightarrow sp^2$ 4 $\rightarrow sp^3$ Bond Length: $sp-C > sp^2-C > sp^3-C$. (More s-character, shorter bond) Bond Strength: $sp-C Electronegativity: $sp > sp^2 > sp^3$. (More s-character, more electronegative) 2. Inductive Effect (I-Effect) Permanent displacement of $\sigma$ electrons along a saturated carbon chain due to difference in electronegativity. Operates through $\sigma$ bonds. Decreases rapidly with distance (negligible after 3-4 carbon atoms). $+I$ Groups (Electron Donating): Alkyl groups. Order: $3^\circ > 2^\circ > 1^\circ$. e.g., $-\text{CH}_3 Also: $-\text{COO}^- > -\text{O}^- > -\text{CH}_2^- > -\text{NH}_2 > -\text{OH}$ $-I$ Groups (Electron Withdrawing): Electronegative atoms/groups. Order: $-\text{NO}_2 > -\text{CN} > -\text{SO}_3\text{H} > -\text{CHO} > -\text{COOR} > -\text{COOH} > -\text{F} > -\text{Cl} > -\text{Br} > -\text{I} > -\text{OH} > -\text{OR} > -\text{NH}_2 > -\text{C}_6\text{H}_5 > -\text{H}$ Applications: Acidic Strength: Increases with $-I$ effect, decreases with $+I$ effect. Basic Strength: Increases with $+I$ effect, decreases with $-I$ effect. Carbocation Stability: Increases with $+I$ effect. Carbanion Stability: Increases with $-I$ effect. 3. Resonance Effect (R-Effect) / Mesomeric Effect (M-Effect) Permanent electron displacement involving $\pi$ electrons or lone pairs in conjugated systems. Operates through $\pi$ bonds. Requires conjugation: alternating single and double bonds, or a lone pair/empty orbital adjacent to a $\pi$ bond. Resonance structures are hypothetical; the actual molecule is a resonance hybrid. Resonance energy = (Energy of resonance hybrid) - (Energy of most stable canonical structure). Conditions for Resonance: All atoms in conjugation must be coplanar. Delocalization of $\pi$ electrons. $+R$ / $+M$ Groups (Electron Donating): Lone pair donating groups. $-\text{NH}_2 > -\text{NHR} > -\text{NR}_2 > -\text{OH} > -\text{OR} > -\text{O}^- > -\text{NHCOCH}_3 > -\text{OCOCH}_3 > -\text{C}_6\text{H}_5 > -\text{X}$ (halogens) Halogens are $-I$ but $+R$. $+R$ effect is weaker than $-I$ effect for halogens. $-R$ / $-M$ Groups (Electron Withdrawing): Electron withdrawing groups through $\pi$ bonds. $-\text{NO}_2 > -\text{CN} > -\text{SO}_3\text{H} > -\text{CHO} > -\text{COR} > -\text{COOH} > -\text{COOR} > -\text{CONH}_2$ Applications: Stability: Greater the number of equivalent resonance structures, greater the stability. Acidity/Basicity: Similar to I-effect, but R-effect is stronger. Electrophilic/Nucleophilic Substitution: Directs incoming groups. Rules for Stability of Resonance Structures: More covalent bonds, more stable. Less charge separation, more stable. Negative charge on more electronegative atom, positive charge on less electronegative atom, more stable. Structures with complete octets are more stable. 4. Hyperconjugation (No-Bond Resonance) Stabilizing interaction involving delocalization of $\sigma$ electrons of C-H bond with an adjacent empty p-orbital or $\pi$ orbital. Operates through $\sigma$ electrons. Requires $\alpha$-hydrogens. Conditions: Carbocation: Carbon adjacent to positively charged carbon must have H-atoms. Alkene: Carbon adjacent to C=C must have H-atoms. Alkyl radical: Carbon adjacent to radical carbon must have H-atoms. Stability Order: Carbocations: $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ (due to hyperconjugation) Alkenes: More substituted alkene is more stable (due to hyperconjugation) Alkyl Radicals: $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ Number of hyperconjugative structures = (Number of $\alpha$-hydrogens) + 1. 5. Aromaticity (Hückel's Rule) Conditions for Aromaticity: Cyclic structure. Planar structure (all atoms $sp^2$ or $sp$ hybridized). Complete conjugation (delocalization of $\pi$ electrons over the entire ring). $(4n+2)\pi$ electrons ($n=0, 1, 2, ...$) (Hückel's Rule). Anti-aromatic Compounds: Cyclic. Planar. Complete conjugation. $4n\pi$ electrons ($n=1, 2, 3, ...$). Highly unstable. Non-aromatic Compounds: Do not satisfy any of the above conditions (e.g., non-planar, not fully conjugated). Examples: Aromatic: Benzene (6$\pi$), Pyridine (6$\pi$), Furan (6$\pi$), Pyrrole (6$\pi$), Thiophene (6$\pi$), Cyclopentadienyl anion (6$\pi$), Cycloheptatrienyl cation (6$\pi$). Anti-aromatic: Cyclobutadiene (4$\pi$), Cyclooctatetraene (8$\pi$, non-planar). 6. Heat of Hydrogenation ($\Delta H_{hydro}$) Energy released when one mole of an unsaturated compound is completely hydrogenated. Reaction: $\text{Alkene} + \text{H}_2 \xrightarrow{\text{catalyst}} \text{Alkane} + \text{Heat}$ Relation to Stability: Lower (less negative) $\Delta H_{hydro}$ implies higher stability of the unsaturated compound. More stable alkene $\implies$ less energy released upon hydrogenation. Factors Affecting $\Delta H_{hydro}$: Number of $\pi$ bonds: Generally, more $\pi$ bonds $\implies$ more negative $\Delta H_{hydro}$ (e.g., alkyne $>$ alkene). Substituent effect: More substituted alkenes are more stable (due to hyperconjugation), thus have lower $\Delta H_{hydro}$ per $\pi$ bond. Stability of alkenes: Tetrasubstituted $>$ Trisubstituted $>$ Disubstituted (trans $>$ cis) $>$ Monosubstituted $>$ Ethene. $\Delta H_{hydro}$ (magnitude) order is reverse of stability. Resonance stabilization: Conjugated dienes are more stable than isolated dienes, so their $\Delta H_{hydro}$ is less negative than expected for two isolated double bonds. Ring strain: Compounds with higher ring strain (e.g., cyclopropene) have higher $\Delta H_{hydro}$. Benzene Exception: Observed $\Delta H_{hydro}$ for benzene is much less negative than 3 times the $\Delta H_{hydro}$ of cyclohexene. The difference is its resonance energy (stability). 7. Heat of Combustion ($\Delta H_{comb}$) Energy released when one mole of a substance is completely burnt in excess oxygen. Reaction: $\text{Hydrocarbon} + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O} + \text{Heat}$ Relation to Stability: Lower (less negative) $\Delta H_{comb}$ implies higher stability of the compound. More stable isomer $\implies$ less energy released upon combustion. Factors Affecting $\Delta H_{comb}$: Number of carbon atoms: Generally, more carbon atoms $\implies$ more negative $\Delta H_{comb}$. Branching: For isomers, branched alkanes are more stable than straight-chain alkanes. Therefore, branched alkanes have lower (less negative) $\Delta H_{comb}$ than their straight-chain isomers. Ring strain: Cyclic compounds with higher ring strain (e.g., cyclopropane, cyclobutane) have higher $\Delta H_{comb}$ per $\text{CH}_2$ unit. Comparison of Isomers: $\Delta H_{comb}$ is used to compare the relative stability of isomers (same molecular formula, different structures). The more stable isomer will have a lower (less negative) heat of combustion. Example: $n$-pentane has a more negative $\Delta H_{comb}$ than neopentane (2,2-dimethylpropane) because neopentane is more stable due to branching. 8. Rotation Energy Barrier Energy required to overcome steric hindrance or electronic repulsion during rotation around a single bond. Determines the relative stability of different conformations (rotamers). Ethane: Eclipsed conformation has higher energy (less stable) due to torsional strain (repulsion between electron clouds of C-H bonds). Staggered conformation has lower energy (more stable) as C-H bonds are maximally separated. Energy difference (barrier) is approx. $12.5 \text{ kJ/mol}$ for ethane. Butane (along C2-C3 bond): Anti-periplanar (anti) is most stable (methyl groups 180° apart). Gauche is less stable than anti due to gauche butane interaction (methyl groups 60° apart). Eclipsed (methyl groups directly opposing) is least stable due to steric hindrance. The energy barrier to rotation is higher than ethane due to larger methyl groups. Factors Increasing Barrier: Larger substituents (steric hindrance). Dipole-dipole interactions. Hydrogen bonding (can stabilize certain conformations). Consequences: At room temperature, rotation around single bonds is generally fast, leading to rapid interconversion of conformers. However, if the barrier is very high, conformers can be isolated (atropisomerism). 9. Dipole Moment ($\mu$) A measure of the polarity of a molecule, resulting from the uneven distribution of electron density. Vector quantity. $\mu = q \times d$, where $q$ is the magnitude of the charge and $d$ is the distance between charges. Unit: Debye (D). Bond Dipole: Arises from difference in electronegativity between two bonded atoms. Molecular Dipole: Vector sum of all individual bond dipoles and lone pair contributions. Factors: Electronegativity Difference: Larger difference $\implies$ larger bond dipole. Molecular Geometry: Symmetrical molecules can have zero net dipole moment even with polar bonds (e.g., $\text{CO}_2$, $\text{CCl}_4$). Lone Pairs: Lone pairs contribute to the overall dipole moment (e.g., $\text{NH}_3$ has a larger dipole moment than $\text{NF}_3$ due to the direction of lone pair dipole). Applications: Polarity: Higher dipole moment $\implies$ more polar molecule. Solubility: "Like dissolves like". Polar solvents dissolve polar solutes. Boiling Point: Polar molecules usually have higher boiling points due to stronger intermolecular forces (dipole-dipole interactions). Distinguishing Isomers: cis -isomers often have a net dipole moment, while trans -isomers can have zero dipole moment if symmetrical. (e.g., cis -1,2-dichloroethene vs trans -1,2-dichloroethene). para -disubstituted benzenes often have zero dipole moment if substituents are identical or cancel out, while ortho / meta isomers have non-zero dipole moments. Resonance Structures: Dipole moments can indicate the contribution of charge-separated resonance structures. 10. Solvation Effect Interaction of a solute with the solvent, leading to stabilization of the solute species. Important for acid-base strength in solution. Basicity of Amines in Aqueous Solution: In gas phase: $3^\circ > 2^\circ > 1^\circ > \text{NH}_3$ (due to $+I$ effect). In aqueous phase: Solvation by $\text{H}_2\text{O}$ stabilizes the conjugate acid (protonated amine). $1^\circ$ amines can form 3 H-bonds, $2^\circ$ amines 2 H-bonds, $3^\circ$ amines 1 H-bond. Combined effect of $+I$ and solvation leads to: Methyl amines: $2^\circ > 1^\circ > 3^\circ > \text{NH}_3$ Ethyl amines: $2^\circ > 3^\circ > 1^\circ > \text{NH}_3$ (steric hindrance becomes more significant for $3^\circ$ with larger alkyl groups) Acidity: Solvation of the conjugate base stabilizes it, increasing acidity. 11. Amine Flipping (Nitrogen Inversion) Rapid interconversion of the two enantiomeric pyramidal forms of a trivalent amine. The nitrogen atom passes through a planar $sp^2$ transition state. Very low energy barrier (approx. $25 \text{ kJ/mol}$), so it occurs rapidly at room temperature. Consequence: Chiral amines with only one chiral center at nitrogen (e.g., $\text{CH}_3\text{CH}_2\text{CH}_3\text{N}$) cannot be resolved into enantiomers at room temperature. If nitrogen is part of a rigid ring system or if the substituents are bulky, inversion can be restricted or prevented. 12. Acid-Base Equilibrium & Solubility General Rule: Stronger acid reacts with stronger base to form weaker acid and weaker base. Solubility in $\text{NaOH}$ (a strong base): Compounds with $pK_a Examples: Carboxylic acids ($pK_a \approx 4-5$), phenols ($pK_a \approx 10$), $\beta$-dicarbonyl compounds ($pK_a \approx 9-13$). Alcohols ($pK_a \approx 16-18$) are generally insoluble in $\text{NaOH}$. Solubility in $\text{NaHCO}_3$ (a weak base): Compounds with $pK_a This is a test for carboxylic acids. Phenols ($pK_a \approx 10$) are generally insoluble in $\text{NaHCO}_3$. Reaction: $\text{RCOOH} + \text{NaHCO}_3 \rightarrow \text{RCOONa} + \text{H}_2\text{O} + \text{CO}_2$ (evolution of $\text{CO}_2$ gas). Amines (Basic compounds): Dissolve in dilute acids (e.g., $\text{HCl}$) by forming water-soluble ammonium salts. 13. Acidic Strength Ability to donate a proton ($H^+$). $K_a \uparrow \implies \text{Acidic strength} \uparrow$. $pK_a \downarrow \implies \text{Acidic strength} \uparrow$. Factors: Electronegativity: Across a period, acidity increases with EN (e.g., $\text{CH}_4 Size: Down a group, acidity increases with size (e.g., $\text{HF} Inductive Effect: $-I$ groups increase acidity, $+I$ groups decrease acidity. Resonance Effect: Electron withdrawal by $-R$ groups increases acidity by stabilizing conjugate base. Electron donation by $+R$ groups decreases acidity. Hybridization: $sp > sp^2 > sp^3$ for C-H bond acidity (due to EN). Stability of Conjugate Base: More stable conjugate base, stronger acid. Carboxylic Acids vs. Phenols vs. Alcohols: $\text{RCOOH} > \text{PhOH} > \text{ROH}$. Carboxylate ion is resonance stabilized by two equivalent structures. Phenoxide ion is resonance stabilized by non-equivalent structures. Alkoxide ion has no resonance stabilization. Substituted Benzoic Acids: $-I$/$-R$ at ortho/para positions increase acidity. $+I$/$+R$ at ortho/para positions decrease acidity. Ortho effect: Ortho substituted benzoic acids are generally stronger acids than benzoic acid itself, regardless of nature of substituent (steric inhibition of resonance of $-\text{COOH}$ with ring). Substituted Phenols: $-I$/$-R$ at ortho/para positions increase acidity. e.g., Picric acid ($2,4,6$-trinitrophenol) is very acidic. $+I$/$+R$ at ortho/para positions decrease acidity. Dicarboxylic Acids: First dissociation constant ($K_{a1}$) is higher for shorter chain lengths due to proximity of $-\text{COOH}$ groups. 14. Basic Strength Ability to accept a proton ($H^+$) or donate a lone pair. $K_b \uparrow \implies \text{Basic strength} \uparrow$. $pK_b \downarrow \implies \text{Basic strength} \uparrow$. Factors: Availability of lone pair: More available, more basic. Inductive Effect: $+I$ groups increase basicity, $-I$ groups decrease basicity. Resonance Effect: If lone pair is involved in resonance, basicity decreases. Hybridization: $sp^3 > sp^2 > sp$ (for N-atom basicity) as s-character increases EN, making lone pair less available. Stability of Conjugate Acid: More stable conjugate acid, stronger base. Amines: Gas Phase: $3^\circ > 2^\circ > 1^\circ > \text{NH}_3$ (only $+I$ effect). Aqueous Phase: As discussed in Solvation Effect ($\uparrow$ H-bonding $\implies \uparrow$ stability of conjugate acid $\implies \uparrow$ basicity). Methyl amines: $2^\circ > 1^\circ > 3^\circ > \text{NH}_3$ Ethyl amines: $2^\circ > 3^\circ > 1^\circ > \text{NH}_3$ Aromatic Amines: Anilines are less basic than aliphatic amines because the lone pair on nitrogen is delocalized into the benzene ring via resonance. Guanidine: Exceptionally strong base due to extensive resonance stabilization of its conjugate acid. 15. Tautomerism Special type of functional isomerism where isomers exist in dynamic equilibrium. Involves migration of a proton and rearrangement of single and double bonds. Most common: Keto-enol tautomerism. Conditions for Keto-enol Tautomerism: Presence of an $\alpha$-hydrogen atom with respect to a carbonyl group. Stability Order: Keto form is usually more stable than enol form due to stronger C=O bond than C=C and O-H bonds. Exceptions (Enol form is more stable): If enol form is stabilized by intramolecular H-bonding (e.g., $\beta$-dicarbonyl compounds like acetylacetone). If enol form is aromatic (e.g., phenol is an enol of cyclohexadienone). 16. Isomerism Compounds having the same molecular formula but different physical and chemical properties. Structural Isomerism: Same molecular formula, different connectivity. Chain: Different carbon skeleton. Position: Different position of functional group/substituent/multiple bond. Functional: Different functional groups. Metamerism: Different alkyl groups attached to the same functional group (e.g., ethers, ketones). Tautomerism: Dynamic equilibrium between two functional isomers. Stereoisomerism: Same molecular formula, same connectivity, different spatial arrangement. Conformational: Interconvertible by rotation around C-C single bonds (e.g., Newman projections, Sawhorse projections). Staggered is more stable than eclipsed. Anti is more stable than gauche. Configurational: Interconversion requires bond breaking. Geometric (Cis-Trans): Due to restricted rotation around a double bond or in a cyclic structure. Conditions: Each carbon of the double bond must be attached to two different groups. E/Z notation for complex cases. Optical: Due to chiral center (asymmetric carbon). Chiral Center: Carbon atom bonded to four different groups. Enantiomers: Non-superimposable mirror images. Rotate plane-polarized light in opposite directions. Identical physical properties except optical activity. Diastereomers: Stereoisomers that are not mirror images. Different physical and chemical properties. Meso Compounds: Contain chiral centers but are optically inactive due to internal plane of symmetry. Racemic Mixture: Equimolar mixture of enantiomers. Optically inactive due to external compensation. Specific Rotation: $[\alpha]_D^T = \frac{\alpha}{l \times c}$ ($\alpha$ = observed rotation, $l$ = path length in dm, $c$ = concentration in g/mL). R/S Configuration: Cahn-Ingold-Prelog rules for assigning absolute configuration. 17. Reaction Intermediates Carbocations: Positively charged carbon with 6 valence electrons, $sp^2$ hybridized, trigonal planar. Stability: $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ (Hyperconjugation & $+I$ effect). Rearrangements: Hydride shift, alkyl shift, ring expansion/contraction to form more stable carbocation. Carbanions: Negatively charged carbon with 8 valence electrons, $sp^3$ (pyramidal) or $sp^2$ (planar). Stability: $\text{methyl} > 1^\circ > 2^\circ > 3^\circ$ (opposite to carbocation due to $-I$ effect). Stabilized by $-I$, $-R$ groups and increased s-character. Free Radicals: Neutral species with an unpaired electron, 7 valence electrons, $sp^2$ hybridized (planar). Stability: $3^\circ > 2^\circ > 1^\circ > \text{methyl}$ (Hyperconjugation & $+I$ effect). Carbenes: Neutral species with a divalent carbon atom having 6 valence electrons (2 bond pairs, 1 lone pair). Singlet carbene: Lone pair in one $sp^2$ orbital, empty p-orbital. Diamagnetic. Reacts stereospecifically in addition. Triplet carbene: Two unpaired electrons in two different p-orbitals. Paramagnetic. Reacts non-stereospecifically. Nitrenes: Nitrogen analog of carbenes. 18. Types of Organic Reactions Substitution: One atom/group replaced by another. Nucleophilic (e.g., $\text{S}_{\text{N}}1, \text{S}_{\text{N}}2$) Electrophilic (e.g., EAS) Free Radical (e.g., halogenation of alkanes) Addition: Atoms/groups added across a multiple bond. Electrophilic (e.g., $\text{HBr}$ to alkene, Markovnikov's rule) Nucleophilic (e.g., $\text{HCN}$ to aldehyde/ketone) Free Radical (e.g., $\text{HBr}$ to alkene in presence of peroxide, anti-Markovnikov) Elimination: Atoms/groups removed to form a multiple bond. $\text{E}1, \text{E}2$ (Saytzeff's rule for major product) Rearrangement: Atoms/groups migrate within the same molecule. 19. Miscellaneous Rules & Concepts Bredt's Rule: A double bond cannot be placed at a bridgehead position in a bicyclic system unless the rings are large enough. This is due to the inability of the bridgehead carbon to adopt a planar $sp^2$ geometry because of ring strain. Fries Rule: States that in polycyclic aromatic hydrocarbons, the most stable resonance structure is the one with the maximum number of benzene rings. Saytzeff's Rule (Zaitsev's Rule): In an elimination reaction, the major product is the more substituted alkene (i.e., the one with the fewer hydrogens on the carbon-carbon double bond). This is generally the more stable alkene. Hofmann's Rule: In certain elimination reactions (e.g., Hofmann elimination of quaternary ammonium salts), the major product is the least substituted alkene. This occurs when the leaving group is very bulky or the base is sterically hindered. Markovnikov's Rule: In the addition of an unsymmetrical reagent ($\text{HX}$) to an unsymmetrical alkene, the hydrogen atom adds to the carbon atom of the double bond that already has more hydrogen atoms. The electrophile adds to the carbon that forms the more stable carbocation. Anti-Markovnikov's Addition: Occurs in the presence of peroxides for $\text{HBr}$ addition to alkenes, where hydrogen adds to the carbon with fewer hydrogens. Involves a free radical mechanism. 20. Common Mistakes & JEE Traps Confusing $+I$/$-I$ with $+R$/$-R$ effects. Remember R-effect is stronger than I-effect (except halogens for acidity/basicity). Not checking for planarity in aromaticity. Cyclooctatetraene is not planar, hence non-aromatic. Ignoring Ortho Effect for substituted benzoic acids. Incorrectly applying Markovnikov's rule (e.g., free radical addition of $\text{HBr}$ is anti-Markovnikov). Overlooking rearrangements in carbocation reactions. Assuming all compounds with chiral centers are optically active (e.g., meso compounds). Not considering solvation effects in basicity of amines in aqueous solution. Forgetting that specific rotation depends on temperature and wavelength of light. Comparing stability of isomers using heat of combustion: More stable isomer has less negative $\Delta H_{comb}$. Comparing stability of unsaturated compounds using heat of hydrogenation: More stable alkene has less negative $\Delta H_{hydro}$. For dipole moment, remember that molecular geometry is critical for overall net dipole moment. Symmetrical molecules can have zero dipole moment even with polar bonds. For rotation energy barrier, understand that steric hindrance is the primary factor, leading to different stabilities of conformers. Applying Bredt's rule incorrectly for bridgehead double bonds in small ring systems. Confusing Saytzeff's and Hofmann's rules for elimination products.