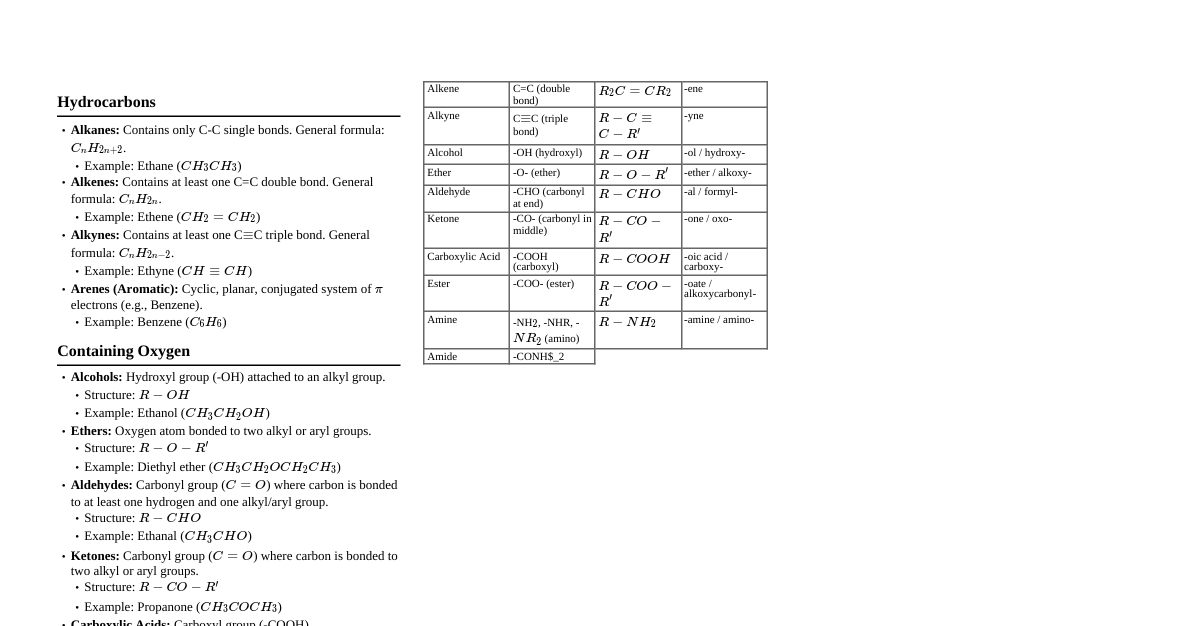
IUPAC Naming Rules Identify the longest continuous carbon chain: This is the parent chain. If there's a functional group, ensure the parent chain includes it. Identify the main functional group: Determine the highest priority functional group (see priority list below). This group dictates the suffix of the parent chain. Number the parent chain: Start numbering from the end that gives the lowest number to the highest priority functional group. If functional groups are absent, number to give substituents the lowest possible numbers. For alkenes/alkynes, give the multiple bond the lowest possible number. Identify and name substituents: Alkyl groups: methyl (-CH$_3$), ethyl (-CH$_2$CH$_3$), propyl (-CH$_2$CH$_2$CH$_3$), isopropyl, butyl, etc. Halogens: fluoro (-F), chloro (-Cl), bromo (-Br), iodo (-I). Other common prefixes: nitro (-NO$_2$), methoxy (-OCH$_3$), amino (-NH$_2$). Alphabetize substituents: List substituents in alphabetical order (ignoring prefixes like di-, tri-, sec-, tert-). Combine into a single name: Use hyphens to separate numbers and words (e.g., 2-methyl). Use commas to separate numbers (e.g., 2,3-dimethyl). Use prefixes (di-, tri-, tetra-) for multiple identical substituents. Functional Group Naming Priority (Highest to Lowest) Carboxylic Acids (-COOH) - Suffix: -oic acid / Prefix: carboxy- Esters (-COOR) - Suffix: -oate / Prefix: alkoxycarbonyl- Acid Halides (-COX) - Suffix: -oyl halide / Prefix: halocarbonyl- Amides (-CONH$_2$) - Suffix: -amide / Prefix: carbamoyl- Nitriles (-C$\equiv$N) - Suffix: -nitrile / Prefix: cyano- Aldehydes (-CHO) - Suffix: -al / Prefix: oxo- (or formyl-) Ketones (-CO-) - Suffix: -one / Prefix: oxo- Alcohols (-OH) - Suffix: -ol / Prefix: hydroxy- Phenols (Ar-OH) - Suffix: -ol / Prefix: hydroxy- Thiols (-SH) - Suffix: -thiol / Prefix: mercapto- Amines (-NH$_2$, -NHR, -NR$_2$) - Suffix: -amine / Prefix: amino- Alkenes (C=C) - Suffix: -ene / Prefix: alkenyl- Alkynes (C$\equiv$C) - Suffix: -yne / Prefix: alkynyl- Ethers (-OR) - Prefix: alkoxy- Halides (-X) - Prefix: halo- (fluoro, chloro, bromo, iodo) Alkanes (-ane) - Suffix: -ane / Prefix: alkyl- The highest priority group determines the suffix of the parent chain. Other groups are named as prefixes. IUPAC Naming Examples Compound: CH$_3$CH(OH)CH$_2$CH$_3$ -> Butan-2-ol Compound: CH$_3$CH(CH$_3$)CH$_2$COOH -> 3-Methylbutanoic acid Compound: CH$_3$CH=CHCH$_2$CH$_2$CHO -> Hex-4-enal Compound: CH$_3$CH$_2$COCH$_2$CH$_3$ -> Pentan-3-one Compound: CH$_3$CH(Cl)CH$_2$CH$_2$NH$_2$ -> 4-Chlorobutan-1-amine Compound: CH$_3$CH$_2$C$\equiv$CCH$_3$ -> Pent-2-yne Compound: (CH$_3$)$_2$CH-O-CH$_2$CH$_3$ -> 1-Isopropoxyethane Compound: CH$_3$CH$_2$CH(CH$_3$)CH$_2$CH$_2$CH$_3$ -> 3-Methylhexane Compound: CH$_3$CH=CHCH$_2$Br -> 1-Bromobut-2-ene Compound: Benzene ring with a -NO$_2$ and -Cl group, ortho to each other -> 1-Chloro-2-nitrobenzene Compound: CH$_3$COOCH$_2$CH$_3$ -> Ethyl ethanoate Compound: (CH$_3$)$_3$N -> Trimethylamine Compound: Cyclohexane with an ethyl group and a methyl group on adjacent carbons -> 1-Ethyl-2-methylcyclohexane Compound: CH$_3$CH(F)CH$_2$CH$_2$CH$_3$ -> 2-Fluoropentane Compound: CH$_3$CH$_2$CH$_2$CONH$_2$ -> Propanamide Hybridization and Geometry Hybridization Geometry Bond Angle Example $sp^3$ Tetrahedral $109.5^\circ$ Methane ($CH_4$) $sp^2$ Trigonal Planar $120^\circ$ Ethene ($C_2H_4$) $sp$ Linear $180^\circ$ Ethyne ($C_2H_2$) Functional Groups Alkane: C-C single bonds Alkene: C=C double bond Alkyne: C$\equiv$C triple bond Arene: Benzene ring Alcohol: -OH (hydroxyl) Ether: R-O-R' Aldehyde: -CHO (carbonyl at end) Ketone: R-CO-R' (carbonyl in middle) Carboxylic Acid: -COOH (carboxyl) Ester: -COOR' Amine: -NH$_2$, -NHR, -NR$_2$ (amino) Amide: -CONR$_2$ Nitrile: -C$\equiv$N (cyano) Thiol: -SH (sulfhydryl) Isomerism Constitutional Isomers Same molecular formula, different connectivity. Example: Butane and Isobutane ($C_4H_{10}$) Stereoisomers Same connectivity, different spatial arrangement. Enantiomers: Non-superimposable mirror images (chiral center). Diastereomers: Stereoisomers that are not mirror images (e.g., cis/trans isomers, multiple chiral centers). Meso Compounds: Contain chiral centers but are achiral due to an internal plane of symmetry. Reaction Types Substitution Reactions $S_N1$ (Unimolecular Nucleophilic Substitution): Two steps: carbocation formation, then nucleophilic attack. Rate = $k[RX]$. Favored by tertiary alkyl halides, weak nucleophiles, protic solvents. Racemization occurs. $S_N2$ (Bimolecular Nucleophilic Substitution): One step: concerted nucleophilic attack and leaving group departure. Rate = $k[RX][Nu]$. Favored by primary alkyl halides, strong nucleophiles, aprotic solvents. Inversion of configuration (Walden inversion). Elimination Reactions $E1$ (Unimolecular Elimination): Two steps: carbocation formation, then deprotonation. Rate = $k[RX]$. Favored by tertiary alkyl halides, weak bases, protic solvents. Zaitsev's rule applies (most substituted alkene is major product). $E2$ (Bimolecular Elimination): One step: concerted deprotonation and leaving group departure. Rate = $k[RX][Base]$. Favored by primary/secondary/tertiary alkyl halides, strong bases, aprotic solvents. Anti-periplanar geometry required for $\beta$-hydrogen and leaving group. Addition Reactions (Alkenes/Alkynes) Hydrohalogenation: H-X adds across double bond. Markovnikov's rule: H adds to carbon with more H's. Hydration: H$_2$O/H$_2$SO$_4$ adds -H and -OH. Markovnikov. Halogenation: X$_2$ adds across double bond (anti-addition). Hydrogenation: H$_2$/Pd, Pt, Ni adds 2 H's (syn-addition). Hydroboration-Oxidation: $BH_3 \cdot THF$, then $H_2O_2, NaOH$. Anti-Markovnikov, syn-addition of H and OH. Carbonyl Reactions Nucleophilic Addition (Aldehydes/Ketones): Grignard reagents (RMgX) + carbonyl $\rightarrow$ alcohol. Hydride reduction ($NaBH_4$ or $LiAlH_4$) $\rightarrow$ alcohol. Cyanohydrin formation (HCN). Nucleophilic Acyl Substitution (Carboxylic Acid Derivatives): Esters, amides, acid chlorides, acid anhydrides. Relative reactivity: Acid Chloride > Anhydride > Ester $\approx$ Carboxylic Acid > Amide. Spectroscopy Nuclear Magnetic Resonance (NMR) $^1H$ NMR: Chemical Shift ($\delta$): Position of signal, indicates electronic environment. Integration: Number of protons represented by signal, provides relative number of equivalent protons. Multiplicity (Splitting): $(n+1)$ rule, where $n$ is number of equivalent adjacent protons. (e.g., singlet, doublet, triplet, quartet). Common Shifts: Alkyl protons: $0.5-2.0$ ppm Allylic/Benzylic protons: $1.5-2.5$ ppm Protons next to -O, -N, -X: $2.5-4.5$ ppm Vinyl protons: $4.5-6.0$ ppm Aromatic protons: $6.0-8.0$ ppm Aldehyde protons: $9.0-10.0$ ppm Carboxylic acid protons: $10.0-12.0$ ppm $^{13}C$ NMR: Chemical Shift ($\delta$): Indicates different types of carbon atoms. Typically no splitting (proton decoupled), unless specifically run with coupling. Common Shifts: Alkyl carbons: $0-50$ ppm Alkyne carbons: $60-90$ ppm Alkene carbons: $100-150$ ppm Aromatic carbons: $120-160$ ppm Carbonyl carbons (ketone/aldehyde): $190-220$ ppm Carboxylic acid/ester carbons: $160-180$ ppm Infrared (IR) Spectroscopy Identifies functional groups based on bond vibrations. Key Absorptions: $O-H$ (alcohol): $3200-3600$ cm$^{-1}$ (broad) $O-H$ (carboxylic acid): $2500-3300$ cm$^{-1}$ (very broad) $N-H$: $3300-3500$ cm$^{-1}$ (medium, 1 or 2 peaks) $C-H$ (sp$^3$): $2850-2960$ cm$^{-1}$ (strong) $C-H$ (sp$^2$): $3020-3100$ cm$^{-1}$ (medium) $C-H$ (sp): $3300$ cm$^{-1}$ (strong, sharp) $C=O$: $1650-1780$ cm$^{-1}$ (strong) $C=C$: $1620-1680$ cm$^{-1}$ (medium) $C\equiv C$: $2100-2260$ cm$^{-1}$ (weak/medium) $C\equiv N$: $2210-2260$ cm$^{-1}$ (medium) Acids and Bases Brønsted-Lowry: Acid donates $H^+$, Base accepts $H^+$. Lewis: Acid accepts electron pair, Base donates electron pair. Factors Affecting Acidity (CARIO): C harge: Positive charge increases acidity. A tom: Electronegativity (across period) and Size (down group) of atom bearing the negative charge. R esonance: Delocalization of negative charge increases stability of conjugate base. I nductive Effect: Electron-withdrawing groups stabilize conjugate base. O rbital: $sp > sp^2 > sp^3$ (more s-character, more acidic). pKa Values: Lower pKa = stronger acid. Common Acid Strengths: Carboxylic acids ($pKa \approx 3-5$) Phenols ($pKa \approx 10$) Alcohols ($pKa \approx 16-18$) Amines (conjugate acid $pKa \approx 9-11$) Arrow Pushing Rules Arrows show electron movement. Start from electron source (lone pair, bond) and end at electron sink (atom, bond). Never "push" an arrow from a positive charge. Break bonds to avoid exceeding octet rule (especially for C, N, O, F). Uses and Applications of Organic Compounds Pharmaceuticals: Most drugs are organic compounds (e.g., aspirin, penicillin, ibuprofen). Polymers: Plastics (polyethylene, PVC), rubbers, fibers (nylon, polyester) are large organic molecules. Fuels: Gasoline, natural gas, propane are primarily hydrocarbons (alkanes). Food & Beverages: Carbohydrates, proteins, fats, vitamins, alcohol, flavorings, and preservatives are organic. Cosmetics & Personal Care: Soaps, detergents, perfumes, lotions, dyes. Agrochemicals: Pesticides, herbicides, fertilizers. Dyes & Pigments: Used in textiles, paints, inks. Solvents: Acetone, ethanol, toluene are widely used in industry and labs. Biochemistry: DNA, RNA, proteins, enzymes, hormones are all complex organic molecules essential for life. Multiple Choice Questions with Explanations Which of the following functional groups has the highest priority in IUPAC naming? Ketone Alcohol Carboxylic Acid Aldehyde Answer: C Explanation: According to IUPAC rules, carboxylic acids have the highest priority among the given options, followed by esters, acid halides, amides, nitriles, aldehydes, ketones, and then alcohols. What is the hybridization of the carbon atoms in an alkyne? $sp^3$ $sp^2$ $sp$ $dsp^2$ Answer: C Explanation: Carbon atoms involved in a triple bond (alkyne) form two sigma bonds and two pi bonds. To accommodate these, they undergo $sp$ hybridization, resulting in a linear geometry. Which type of isomerism involves compounds with the same molecular formula but different connectivity? Enantiomers Diastereomers Constitutional Isomers Geometric Isomers Answer: C Explanation: Constitutional isomers (also called structural isomers) differ in the order in which their atoms are connected, leading to different bonding arrangements. Stereoisomers (enantiomers, diastereomers, geometric isomers) have the same connectivity but different spatial arrangements. Which reaction mechanism results in an inversion of configuration at the chiral center? $S_N1$ $S_N2$ $E1$ $E2$ Answer: B Explanation: $S_N2$ reactions proceed via a concerted mechanism where the nucleophile attacks from the backside, leading to a Walden inversion (inversion of configuration) at the chiral center. $S_N1$ reactions involve a carbocation intermediate, leading to racemization. According to Markovnikov's rule, in the hydrohalogenation of an unsymmetrical alkene, the hydrogen atom adds to the carbon atom that: Has fewer hydrogen atoms already attached. Has more hydrogen atoms already attached. Is more sterically hindered. Is part of a triple bond. Answer: B Explanation: Markovnikov's rule states that in the addition of HX to an unsymmetrical alkene, the hydrogen atom adds to the carbon atom of the double bond that already has the greater number of hydrogen atoms, leading to the formation of the more stable carbocation intermediate. A broad signal in the $3200-3600$ cm$^{-1}$ region in an IR spectrum typically indicates the presence of which functional group? Aldehyde ($C=O$) Carboxylic Acid ($C=O$) Alcohol ($O-H$) Alkyne ($C\equiv C$) Answer: C Explanation: The $O-H$ stretching vibration of an alcohol typically appears as a broad band in the $3200-3600$ cm$^{-1}$ range due to hydrogen bonding. Carboxylic acids also have an O-H stretch, but it's even broader and shifted to lower wavenumbers ($2500-3300$ cm$^{-1}$). Which factor primarily contributes to the increased acidity of a carboxylic acid compared to an alcohol? Inductive effect of the alkyl group. Resonance stabilization of the conjugate base. Steric hindrance. Presence of a double bond. Answer: B Explanation: The conjugate base of a carboxylic acid (carboxylate ion) is resonance-stabilized, meaning the negative charge is delocalized over two oxygen atoms. This delocalization greatly stabilizes the conjugate base, making the carboxylic acid more acidic than an alcohol, whose conjugate base (alkoxide ion) has its negative charge localized on a single oxygen atom. What is the correct IUPAC name for CH$_3$CH$_2$CH$_2$CHO? Butanone Butanal Butanoic acid Propanal Answer: B Explanation: The compound has a four-carbon chain with an aldehyde (-CHO) functional group at the end. The suffix for an aldehyde is "-al". Thus, it is butanal. In a $^{13}C$ NMR spectrum, a signal around $190-220$ ppm is characteristic of which type of carbon? Alkyl carbon Aromatic carbon Alkene carbon Carbonyl carbon (ketone/aldehyde) Answer: D Explanation: Carbonyl carbons (C=O) in aldehydes and ketones are highly deshielded due to the electronegativity of oxygen and the $\pi$-bond, causing them to appear at very high chemical shifts, typically in the $190-220$ ppm range. Which of the following organic compounds is a common component of fuels? Proteins Polymers Hydrocarbons Pharmaceuticals Answer: C Explanation: Fuels like gasoline, diesel, and natural gas are primarily composed of hydrocarbons (compounds containing only carbon and hydrogen atoms). What is the geometry around a carbon atom with $sp^2$ hybridization? Tetrahedral Trigonal planar Linear Bent Answer: B Explanation: $sp^2$ hybridized carbon atoms form three sigma bonds and one pi bond. The three sigma bonds repel each other to achieve maximum separation, resulting in a trigonal planar geometry with bond angles of approximately $120^\circ$. Which of the following is an example of an $S_N1$ reaction favoring condition? Primary alkyl halide Strong nucleophile Aprotic solvent Tertiary alkyl halide Answer: D Explanation: $S_N1$ reactions proceed through a carbocation intermediate. Tertiary alkyl halides form the most stable carbocations, thus favoring the $S_N1$ mechanism. Primary alkyl halides and strong nucleophiles favor $S_N2$, while aprotic solvents favor $S_N2$ over $S_N1$ (protic solvents stabilize carbocations for $S_N1$). What is the IUPAC name for CH$_3$CH$_2$COOCH$_3$? Methyl propanoate Ethyl acetate Propyl methanoate Methyl ethanoate Answer: A Explanation: This is an ester. The alkyl group attached to the oxygen is methyl. The acyl part (CH$_3$CH$_2$COO-) comes from propanoic acid. So, it's methyl propanoate. An organic compound that contains a -SH group is classified as a: Alcohol Ether Thiol Amine Answer: C Explanation: The -SH functional group is called a thiol (or mercaptan). Alcohols have -OH, ethers have R-O-R', and amines have -NH$_2$, -NHR, or -NR$_2$. Which rule states that in an elimination reaction, the major product is the most substituted alkene? Markovnikov's Rule Zaitsev's Rule Hofmann's Rule Walden Inversion Answer: B Explanation: Zaitsev's rule (or Saytzeff's rule) states that the major product in an elimination reaction (E1 or E2) is the most substituted (more stable) alkene. Hofmann's rule is the opposite and applies under specific conditions (e.g., bulky bases). Markovnikov's rule applies to addition reactions. A compound with the same molecular formula as another but with a different arrangement of atoms in space (not mirror images) is a: Enantiomer Constitutional isomer Diastereomer Meso compound Answer: C Explanation: Diastereomers are stereoisomers that are not mirror images of each other. Enantiomers are non-superimposable mirror images. Constitutional isomers have different connectivity. Meso compounds are achiral compounds that contain chiral centers. In $^{1}H$ NMR spectroscopy, the 'n+1' rule helps determine the: Chemical shift Integration Multiplicity (splitting) Number of carbon atoms Answer: C Explanation: The 'n+1' rule (where n is the number of equivalent adjacent protons) is used to predict the splitting pattern (multiplicity) of a signal in an $^{1}H$ NMR spectrum. It indicates how many peaks a signal will be split into due to coupling with neighboring protons. Which of the following is a Lewis acid? $NH_3$ $H_2O$ $BF_3$ $OH^-$ Answer: C Explanation: A Lewis acid is an electron pair acceptor. $BF_3$ has an incomplete octet on boron and can accept an electron pair, making it a Lewis acid. $NH_3$, $H_2O$, and $OH^-$ are Lewis bases because they have lone pairs of electrons they can donate. What is the primary product of the reaction of an aldehyde with a Grignard reagent (RMgX) followed by aqueous workup? Ketone Carboxylic Acid Primary Alcohol Secondary Alcohol Answer: D Explanation: Grignard reagents are strong nucleophiles. When they react with an aldehyde, they add to the carbonyl carbon, forming an alkoxide intermediate. Upon aqueous workup, this alkoxide is protonated to yield a secondary alcohol. If formaldehyde (the simplest aldehyde) is used, a primary alcohol is formed. Ketones react to form tertiary alcohols. Which type of organic reaction involves the breaking of a $\pi$-bond and formation of two new $\sigma$-bonds? Substitution Elimination Addition Rearrangement Answer: C Explanation: Addition reactions (e.g., hydrogenation, hydrohalogenation) typically involve the breaking of a less stable $\pi$-bond in an unsaturated compound (like an alkene or alkyne) and the formation of two more stable $\sigma$-bonds as atoms or groups are added across the multiple bond. Which of the following is typically a strong base and a poor nucleophile due to steric hindrance? $CH_3CH_2O^-$ $HO^-$ $(CH_3)_3CO^-$ (tert-butoxide) $CN^-$ Answer: C Explanation: Tert-butoxide is a bulky base. Its large size hinders its ability to act as a nucleophile (attack a carbon center), but it is very effective as a base in abstracting a proton, especially in E2 reactions, leading to the Hofmann product (less substituted alkene). The process of converting an aldehyde or ketone into an alcohol using $NaBH_4$ is an example of: Oxidation Reduction Hydrolysis Substitution Answer: B Explanation: The addition of hydrogen (or equivalent, such as hydride from $NaBH_4$) to a carbonyl group, decreasing the number of bonds to oxygen, is a reduction reaction. Specifically, $NaBH_4$ reduces aldehydes to primary alcohols and ketones to secondary alcohols. What is the common name for the smallest ketone? Propanal Acetone Ethanal Formaldehyde Answer: B Explanation: The smallest ketone has three carbon atoms, with the carbonyl group ($\text{C=O}$) in the middle. Its IUPAC name is propanone, and its common name is acetone. Which type of solvent favors $S_N1$ reactions by stabilizing the carbocation intermediate? Aprotic, polar Protic, polar Aprotic, nonpolar Protic, nonpolar Answer: B Explanation: Polar protic solvents (e.g., water, alcohols) can solvate and stabilize carbocation intermediates through hydrogen bonding, which is crucial for the formation of the carbocation in $S_N1$ reactions. What is the correct order of increasing boiling points for the following compounds: n-butane, propan-1-ol, acetone? n-butane acetone propan-1-ol n-butane Answer: A Explanation: Boiling point is influenced by intermolecular forces. n-butane is an alkane (nonpolar), only has London dispersion forces. Acetone is a ketone (polar), has dipole-dipole interactions. Propan-1-ol is an alcohol, has strong hydrogen bonding. Therefore, the order of increasing boiling points is n-butane (weakest forces) Which statement about Meso compounds is true? They are optically active. They have no chiral centers. They possess an internal plane of symmetry. They are enantiomers of each other. Answer: C Explanation: Meso compounds are achiral (optically inactive) despite having two or more chiral centers because they possess an internal plane of symmetry, which makes the molecule superimposable on its mirror image. The reaction of an alkene with $H_2$ under the presence of a metal catalyst (e.g., Pd, Pt) is called: Hydrohalogenation Hydration Hydrogenation Halogenation Answer: C Explanation: Hydrogenation is the addition of hydrogen ($H_2$) across a double or triple bond, typically catalyzed by transition metals, to form a saturated compound. Which of the following would show a strong absorption around $1700$ cm$^{-1}$ in its IR spectrum? Alkyne Alcohol Amine Ketone Answer: D Explanation: The $C=O$ (carbonyl) stretch typically appears as a strong absorption in the $1650-1780$ cm$^{-1}$ range. Ketones contain a carbonyl group. What is the product when an ester is hydrolyzed in the presence of acid? Alcohol and ether Carboxylic acid and alcohol Amine and carboxylic acid Ketone and alcohol Answer: B Explanation: Acid-catalyzed hydrolysis of an ester cleaves the ester bond, yielding a carboxylic acid and an alcohol. Which of the following is an example of an electron-withdrawing group (EWG) that increases the acidity of a carboxylic acid through inductive effect? $-CH_3$ $-OH$ $-Cl$ $-NH_2$ Answer: C Explanation: Electron-withdrawing groups (EWGs) like halogens (e.g., -Cl) can stabilize the conjugate base of a carboxylic acid by drawing electron density away from the carboxylate group through the sigma bonds, thereby increasing its acidity. Alkyl groups (-CH$_3$) are electron-donating, while -OH and -NH$_2$ can be both electron-donating by resonance and electron-withdrawing by induction, but halogens are primarily EWG by induction. What type of bond is formed when an $sp^2$ hybridized carbon atom overlaps with another $sp^2$ hybridized carbon atom? Single $\sigma$ bond only Double bond ($\sigma$ and $\pi$ bonds) Triple bond ($\sigma$ and two $\pi$ bonds) Ionic bond Answer: B Explanation: When two $sp^2$ hybridized carbon atoms bond, one $sp^2$ orbital from each carbon overlaps head-on to form a sigma ($\sigma$) bond. The remaining unhybridized p-orbitals on each carbon then overlap sideways to form a pi ($\pi$) bond, resulting in a double bond. Which of the following is characteristic of an aromatic compound? It must contain a benzene ring. It must be planar, cyclic, fully conjugated, and have $4n+2$ $\pi$ electrons. It must have alternating single and double bonds. It must be highly reactive towards addition reactions. Answer: B Explanation: Hückel's rule defines aromaticity: the compound must be cyclic, planar, fully conjugated (every atom in the ring has a p-orbital), and contain $(4n+2)$ $\pi$ electrons (where n is a non-negative integer). While benzene is a common aromatic compound, not all aromatic compounds are benzene derivatives. A primary alcohol can be oxidized to a carboxylic acid using: PCC (Pyridinium Chlorochromate) $CrO_3$ (Chromium Trioxide) in water/acid $NaBH_4$ (Sodium Borohydride) $LiAlH_4$ (Lithium Aluminum Hydride) Answer: B Explanation: Strong oxidizing agents like chromium trioxide ($CrO_3$) in acid (Jones reagent) can oxidize primary alcohols directly to carboxylic acids. PCC is a milder oxidizing agent that converts primary alcohols to aldehydes. $NaBH_4$ and $LiAlH_4$ are reducing agents. What type of intermediate is formed during an $E1$ elimination reaction? Carbanion Carbocation Free radical Transition state only Answer: B Explanation: $E1$ (unimolecular elimination) reactions proceed in two steps. The first step, which is rate-determining, involves the departure of the leaving group to form a carbocation intermediate. The second step is the deprotonation of an adjacent carbon by a base. Which spectroscopic technique is most useful for determining the molecular weight and fragmentation pattern of an organic compound? IR Spectroscopy UV-Vis Spectroscopy NMR Spectroscopy Mass Spectrometry Answer: D Explanation: Mass spectrometry (MS) measures the mass-to-charge ratio of ions, providing information about the molecular weight of the compound (from the molecular ion peak) and its fragmentation pattern, which can help in structural elucidation. Which of the following is the correct IUPAC name for a compound with a benzene ring substituted with a methyl group and a hydroxyl group, where the methyl is ortho to the hydroxyl? 2-Methylphenol o-Hydroxytoluene Methylbenzene alcohol 1-Hydroxy-2-methylbenzene Answer: A Explanation: When a benzene ring is substituted with a methyl group and a hydroxyl group, the hydroxyl group (-OH) takes precedence as the principal functional group, making it a phenol derivative. The methyl group is then located relative to the hydroxyl. "o-Hydroxytoluene" is a common name, but 2-Methylphenol is the preferred IUPAC name where the carbon bearing the -OH is designated as C1. What is the function of a catalyst in an organic reaction? To shift the equilibrium towards products. To increase the activation energy of the reaction. To increase the rate of reaction by providing an alternative reaction pathway with lower activation energy. To make a non-spontaneous reaction spontaneous. Answer: C Explanation: Catalysts speed up reactions by lowering the activation energy, but they do not affect the equilibrium position or make a non-spontaneous reaction spontaneous. They simply allow the reaction to reach equilibrium faster. Which of the following statements about free radicals is true? They are highly stable due to resonance. They are typically formed by heterolytic cleavage of a bond. They possess an unpaired electron. They are electrophiles. Answer: C Explanation: Free radicals are species that possess an unpaired electron. They are typically formed by homolytic cleavage of a bond (each atom in the bond takes one electron). They are generally highly reactive, not stable, and act as electrophiles or nucleophiles depending on the reaction. They can be electrophilic (electron-deficient) because of the unpaired electron. What is the major product of the reaction between propyne and $H_2O$ with $H_2SO_4$ and $HgSO_4$ catalysts? Propan-1-ol Propan-2-one Propanal Propene Answer: B Explanation: The hydration of alkynes (using $H_2SO_4$ and $HgSO_4$) follows Markovnikov's rule, initially forming an enol. The enol then rapidly tautomerizes to the more stable keto form. For propyne, the enol is prop-1-en-2-ol, which tautomerizes to propan-2-one (acetone). Ethers are generally considered to be: Highly reactive towards strong acids and bases. Inert and often used as solvents. Strong oxidizing agents. Easily hydrolyzed to alcohols. Answer: B Explanation: Ethers are relatively unreactive compounds due to the stable C-O-C bond. This inertness makes them excellent solvents for many organic reactions, as they do not readily react with common reagents.