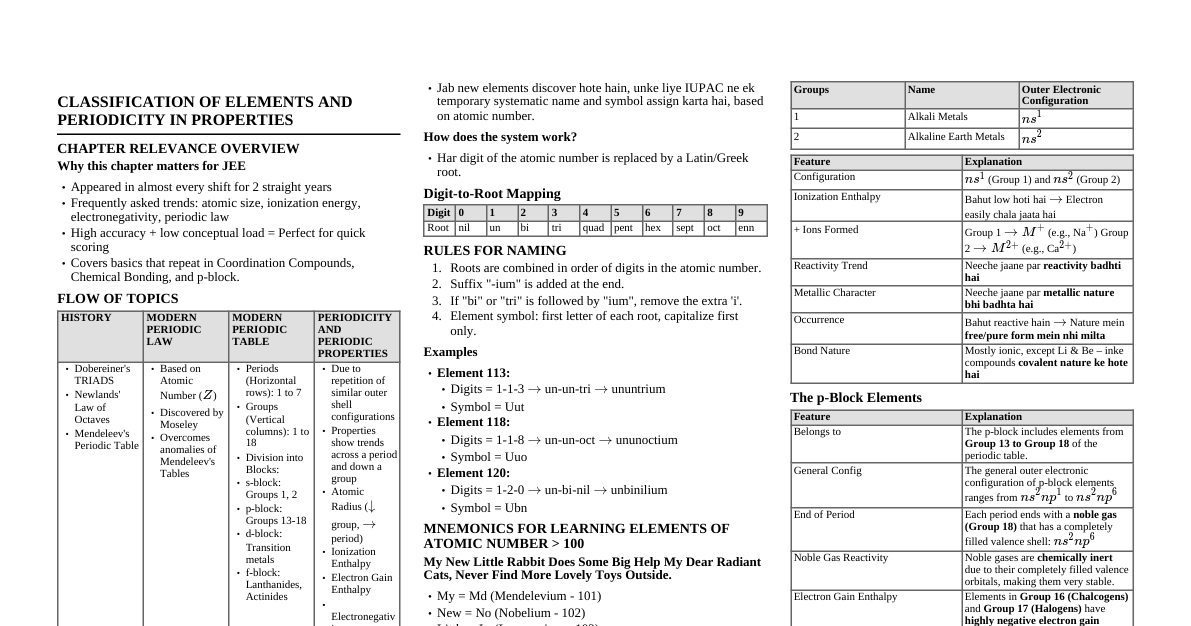
1. Periodic Properties & Variations (i) Definitions and Trends Atomic Size: Distance from nucleus to outermost electron shell. Period: Decreases (due to increasing nuclear charge pulling electrons closer). Group: Increases (due to increasing number of electron shells). Metallic Character: Tendency to lose electrons. Period: Decreases (non-metals increase). Group: Increases (easier to lose valence electrons). Non-metallic Character: Tendency to gain electrons. Period: Increases. Group: Decreases. Ionisation Potential (IE): Energy required to remove an electron from a gaseous atom. Period: Increases (stronger nuclear attraction). Group: Decreases (outermost electrons further from nucleus, less attraction). Electron Affinity (EA): Energy released when an electron is added to a gaseous atom. Period: Increases (atoms become smaller, stronger attraction for added electron). Group: Decreases (atoms become larger, weaker attraction for added electron). Electronegativity: Tendency of an atom to attract shared electron pair in a covalent bond. Period: Increases. Group: Decreases. (ii) Periodicity based on Atomic Number Modern Periodic Table up to Period 3 (Argon). Properties explained by nuclear charge and number of shells. Specific reference to Alkali Metals (Group 1) and Halogens (Group 17). Alkali Metals: Highly reactive, low IE, large atomic size, strong metallic character. Halogens: Highly reactive, high EA & electronegativity, strong non-metallic character. 2. Chemical Bonding (a) Electrovalent Bonding (Ionic) Transfer of electrons (metal to non-metal). Electron Dot Structures: NaCl: $Na^+[:\ddot{Cl}:]^-$ MgCl$_2$: $[:\ddot{Cl}:]^- Mg^{2+} [:\ddot{Cl}:]^-$ CaO: $Ca^{2+}[:\ddot{O}:]^{2-}$ Characteristic Properties: State: Crystalline solids. Melting/Boiling Points: High (strong electrostatic forces). Conductivity: Conduct in molten state or aqueous solution (due to mobile ions), not in solid state. Dissociation: Dissociate into ions in solution/molten state. (b) Covalent Bonding Sharing of electrons (between non-metals). Electron Dot Structures (Duplet/Octet): Hydrogen ($H_2$): $H:H$ Chlorine ($Cl_2$): $:\ddot{Cl}:\ddot{Cl}:$ Nitrogen ($N_2$): $:\text{N}\equiv\text{N}:$ Ammonia ($NH_3$): $H:\underset{\text{H}}{\ddot{N}}:H$ Carbon Tetrachloride ($CCl_4$): $:\ddot{Cl}:\underset{\overset{\cdot\cdot}{Cl}}{\overset{\overset{\cdot\cdot}{Cl}}{\text{C}}}:\ddot{Cl}:$ Methane ($CH_4$): $H:\underset{\overset{H}{\cdot\cdot}}{\overset{\cdot\cdot}{C}}:H$ Polar Covalent Compounds: Unequal sharing due to electronegativity difference. HCl: $H-\ddot{Cl}:$ ($\delta^+H - \delta^-Cl$) H$_2$O: $H-\ddot{O}-H$ ($\delta^+H - \delta^-O - \delta^+H$) Characteristic Properties: State: Gases, liquids, or soft solids. Melting/Boiling Points: Low (weak intermolecular forces). Conductivity: Non-conductors (no free ions/electrons). Ionisation: Do not ionise in solution (except polar ones like HCl). Comparison: Property Electrovalent Covalent Electron Transfer Complete transfer Sharing State Solid Gas, liquid, soft solid M.P./B.P. High Low Conductivity Molten/aq. solution Non-conductor (c) Coordinate Bonding (Dative) Sharing of electron pair, with both electrons donated by one atom (donor). Lone Pair: Non-bonding pair of electrons. Formation of Hydronium Ion ($H_3O^+$): $H_2O + H^+ \rightarrow H_3O^+$ Electron dot: $H:\ddot{O}:H + H^+ \rightarrow [\underset{\text{H}}{\overset{\text{H}}{\cdot\cdot O}}:H]^+$ Formation of Ammonium Ion ($NH_4^+$): $NH_3 + H^+ \rightarrow NH_4^+$ Electron dot: $H:\underset{\text{H}}{\ddot{N}}:H + H^+ \rightarrow [\overset{\text{H}}{\underset{\text{H}}{\text{N}}}:H]^+$ 3. Acids, Bases and Salts (i) Definitions & Properties Acids: Substances that produce $H^+$ ions (hydronium $H_3O^+$) in water. Turn blue litmus red. Bases: Substances that produce $OH^-$ ions in water. Turn red litmus blue. Salts: Ionic compounds formed from the reaction of an acid and a base. (ii) Ions, Litmus & pH Acids: $HCl \rightarrow H^+ + Cl^-$ (or $H_3O^+$). Alkalis: $NaOH \rightarrow Na^+ + OH^-$. Salts: $NaCl \rightarrow Na^+ + Cl^-$. pH Scale: Measures acidity/alkalinity. pH pH = 7: Neutral pH > 7: Alkaline/Basic (iii) Types of Salts Normal Salts: Complete replacement of acidic H by metal. E.g., $Na_2SO_4$. Acid Salts: Partial replacement of acidic H by metal. E.g., $NaHSO_4$. Basic Salts: Partial replacement of hydroxyl (OH) group by acid radical. E.g., $Pb(OH)Cl$. (iv) Action of Dilute Acids on Salts Carbonates/Bicarbonates ($CO_3^{2-}, HCO_3^-$): Produce $CO_2$. $CaCO_3(s) + 2HCl(aq) \rightarrow CaCl_2(aq) + H_2O(l) + CO_2(g)$ Sulphites ($SO_3^{2-}$): Produce $SO_2$. $Na_2SO_3(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H_2O(l) + SO_2(g)$ Sulphides ($S^{2-}$): Produce $H_2S$. $FeS(s) + 2HCl(aq) \rightarrow FeCl_2(aq) + H_2S(g)$ (v) Methods of Preparation of Normal Salts Direct Combination: $Fe + S \xrightarrow{\Delta} FeS$ Displacement: $Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2$ Precipitation (Double Decomposition): $AgNO_3(aq) + NaCl(aq) \rightarrow AgCl(s) + NaNO_3(aq)$ Neutralisation of Insoluble Base: $CuO(s) + H_2SO_4(aq) \rightarrow CuSO_4(aq) + H_2O(l)$ Neutralisation of Alkali (Titration): $NaOH(aq) + HCl(aq) \rightarrow NaCl(aq) + H_2O(l)$ Action of Dilute Acids on Carbonates/Bicarbonates: $MgCO_3(s) + 2HCl(aq) \rightarrow MgCl_2(aq) + H_2O(l) + CO_2(g)$ 4. Analytical Chemistry (i) Action of Ammonium Hydroxide & Sodium Hydroxide on Salt Solutions General Reactions: $M^{2+}(aq) + 2OH^-(aq) \rightarrow M(OH)_2(s)$ (Precipitate) $M^{3+}(aq) + 3OH^-(aq) \rightarrow M(OH)_3(s)$ (Precipitate) Observation Table: Metal Ion Color of Salt Solution NaOH (drop by drop) NaOH (in excess) NH$_4$OH (drop by drop) NH$_4$OH (in excess) Ca$^{2+}$ Colorless White ppt ($Ca(OH)_2$) Insoluble No ppt / slight white ppt No ppt / slight white ppt Fe$^{2+}$ Pale Green Dirty green ppt ($Fe(OH)_2$) Insoluble Dirty green ppt ($Fe(OH)_2$) Insoluble Fe$^{3+}$ Yellow/Brown Reddish-brown ppt ($Fe(OH)_3$) Insoluble Reddish-brown ppt ($Fe(OH)_3$) Insoluble Cu$^{2+}$ Blue Pale blue ppt ($Cu(OH)_2$) Insoluble Pale blue ppt ($Cu(OH)_2$) Deep azure blue solution ($[Cu(NH_3)_4]^{2+}$) Zn$^{2+}$ Colorless White gelatinous ppt ($Zn(OH)_2$) Soluble (colorless solution) ($[Zn(OH)_4]^{2-}$) White gelatinous ppt ($Zn(OH)_2$) Soluble (colorless solution) ($[Zn(NH_3)_4]^{2+}$) Pb$^{2+}$ Colorless White chalky ppt ($Pb(OH)_2$) Soluble (colorless solution) ($[Pb(OH)_4]^{2-}$) White chalky ppt ($Pb(OH)_2$) Insoluble Special Action: $Cu^{2+}$ with $NH_4OH$ (excess): $CuSO_4 + 4NH_4OH \rightarrow [Cu(NH_3)_4]SO_4 + 4H_2O$ (Deep blue solution) $NH_4^+$ with $NaOH$: $NH_4Cl(aq) + NaOH(aq) \xrightarrow{\Delta} NaCl(aq) + H_2O(l) + NH_3(g)$ (Ammonia gas evolved) (ii) Action of Alkalis on Amphoteric Metals/Oxides/Hydroxides Amphoteric substances react with both acids and strong bases. Metals: Al, Zn, Pb $2Al(s) + 2NaOH(aq) + 2H_2O(l) \rightarrow 2NaAlO_2(aq) + 3H_2(g)$ (Sodium aluminate) $Zn(s) + 2NaOH(aq) \rightarrow Na_2ZnO_2(aq) + H_2(g)$ (Sodium zincate) $Pb(s) + 2NaOH(aq) \rightarrow Na_2PbO_2(aq) + H_2(g)$ (Sodium plumbite) Oxides: $Al_2O_3, ZnO, PbO$ $Al_2O_3(s) + 2NaOH(aq) \rightarrow 2NaAlO_2(aq) + H_2O(l)$ $ZnO(s) + 2NaOH(aq) \rightarrow Na_2ZnO_2(aq) + H_2O(l)$ $PbO(s) + 2NaOH(aq) \rightarrow Na_2PbO_2(aq) + H_2O(l)$ Hydroxides: $Al(OH)_3, Zn(OH)_2, Pb(OH)_2$ $Al(OH)_3(s) + NaOH(aq) \rightarrow Na[Al(OH)_4](aq)$ (Sodium tetrahydroxoaluminate(III)) $Zn(OH)_2(s) + 2NaOH(aq) \rightarrow Na_2[Zn(OH)_4](aq)$ (Sodium tetrahydroxozincate(II)) $Pb(OH)_2(s) + 2NaOH(aq) \rightarrow Na_2[Pb(OH)_4](aq)$ (Sodium tetrahydroxoplumbate(II)) 5. Mole Concept and Stoichiometry (i) Gay Lussac's Law; Avogadro's Law; Molar Volume Mole: A number ($6.022 \times 10^{23}$, Avogadro's number). Avogadro's Law: Equal volumes of all gases under the same conditions of temperature and pressure contain an equal number of molecules. Gay Lussac's Law of Combining Volumes: When gases react, they do so in volumes which bear a simple whole number ratio to one another, and to the volumes of the gaseous products, provided that all volumes are measured at the same temperature and pressure. Molar Volume: 1 mole of any gas at S.T.P. ($0^\circ C$, 1 atm) occupies 22.4 L. Calculations: Based on molar volume and combining volumes. (ii) Atomicity Hydrogen ($H_2$), Oxygen ($O_2$), Nitrogen ($N_2$), Chlorine ($Cl_2$) are diatomic. Examples: Formation of HCl, NH$_3$, NO. (iii) Vapour Density & Molecular Mass Molecular Mass = 2 $\times$ Vapour Density Empirical Formula: Simplest whole number ratio of atoms in a compound. Molecular Formula: Actual number of atoms of each element in a molecule. Deduction from percentage composition or masses of combining elements. (iv) Mole and Mass Relation Gram Atomic Mass: Mass of one mole of atoms of an element (in grams). Gram Molecular Mass: Mass of one mole of molecules of a substance (in grams). Calculations: Relating moles to mass, volume (at S.T.P.), and Avogadro's number. Moles = Mass / Molar Mass Moles = Volume (at S.T.P.) / 22.4 L Moles = Number of particles / Avogadro's Number (v) Calculations based on Chemical Equations Stoichiometric calculations involving weights and/or volumes of reactants and products. 6. Electrolysis (i) Electrolytes & Non-electrolytes Electrolytes: Substances that conduct electricity in molten state or aqueous solution (due to mobile ions). E.g., NaCl, $H_2SO_4$. Non-electrolytes: Substances that do not conduct electricity. E.g., Glucose, Urea. (ii) Substances based on Composition Molecules only: Non-electrolytes (e.g., sugar, alcohol). Ions only: Solid ionic compounds (non-conductors). Both molecules and ions: Acids, bases, salts in aqueous solution (electrolytes). Strong Electrolytes: Almost completely dissociate into ions (e.g., strong acids, strong bases, most salts). Weak Electrolytes: Partially dissociate into ions (e.g., weak acids, weak bases). (iii) Definitions Electrolysis: Decomposition of a compound in molten state or aqueous solution by passing electric current. Electrolyte: Substance undergoing electrolysis. Electrode: Conductor through which current enters/leaves electrolyte. Anode: Positive electrode (oxidation occurs). Cathode: Negative electrode (reduction occurs). Anion: Negatively charged ion (moves to anode). Cation: Positively charged ion (moves to cathode). Oxidation: Loss of electrons (at anode). Reduction: Gain of electrons (at cathode). (iv) Migration & Selective Discharge of Ions Factors influencing selective discharge: Position in activity series, concentration of ions, nature of electrodes. 1. Molten Lead Bromide ($PbBr_2$): Electrolyte: Molten $PbBr_2$ Electrodes: Graphite Ions: $Pb^{2+}, Br^-$ Anode (oxidation): $2Br^- \rightarrow Br_2(g) + 2e^-$ (Reddish-brown gas) Cathode (reduction): $Pb^{2+} + 2e^- \rightarrow Pb(l)$ (Silvery-grey metal) 2. Acidified Water ($H_2O$ with trace $H_2SO_4$): Electrolyte: Dilute $H_2SO_4$ Electrodes: Platinum Ions: $H^+, OH^-, SO_4^{2-}$ (from $H_2SO_4$), $H^+, OH^-$ (from $H_2O$) Anode (oxidation): $4OH^- \rightarrow 2H_2O(l) + O_2(g) + 4e^-$ (Oxygen gas) Cathode (reduction): $4H^+ + 4e^- \rightarrow 2H_2(g)$ (Hydrogen gas) 3. Aqueous Copper(II) Sulphate ($CuSO_4$) with Copper Electrodes: Electrolyte: Aqueous $CuSO_4$ Electrodes: Anode (impure Cu), Cathode (pure Cu) Ions: $Cu^{2+}, SO_4^{2-}, H^+, OH^-$ Anode (oxidation): $Cu(s) \rightarrow Cu^{2+}(aq) + 2e^-$ (Anode dissolves) Cathode (reduction): $Cu^{2+}(aq) + 2e^- \rightarrow Cu(s)$ (Pure copper deposits) (v) Applications of Electrolysis Electroplating: Coating a cheaper metal with a thin layer of a more expensive/corrosion-resistant metal. Conditions: Clean article, low current, long time, suitable electrolyte, article as cathode, plating metal as anode. Electroplating with Nickel: Electrolyte - Nickel Sulphate; Anode - Nickel; Cathode - Article. Electroplating with Silver: Electrolyte - Sodium Argentocyanide; Anode - Silver; Cathode - Article. Electrorefining of Copper: Purifying impure copper. Anode: Impure copper block. Cathode: Thin sheet of pure copper. Electrolyte: Copper(II) sulphate solution with dilute $H_2SO_4$. Anode reaction: Impure $Cu \rightarrow Cu^{2+} + 2e^-$ (more reactive metals also oxidize). Cathode reaction: $Cu^{2+} + 2e^- \rightarrow Cu$ (pure copper deposits). 7. Metallurgy (i) Occurrence of Metals Mineral: Naturally occurring chemical substance from which a metal can be extracted economically or not. Ore: Mineral from which a metal can be profitably extracted. Common Ores: Iron: Haematite ($Fe_2O_3$), Magnetite ($Fe_3O_4$) Aluminium: Bauxite ($Al_2O_3 \cdot 2H_2O$) Zinc: Zinc blende ($ZnS$), Calamine ($ZnCO_3$) (ii) Stages in Metal Extraction (a) Dressing of Ore (Concentration): Hydrolytic Method: Washing with water to remove lighter impurities. Magnetic Separation: For magnetic ores (e.g., Magnetite). Froth Flotation: For sulphide ores (e.g., Zinc blende). (b) Conversion of Concentrated Ore to Oxide: Roasting: Heating sulphide ores in excess air (e.g., $2ZnS + 3O_2 \xrightarrow{\Delta} 2ZnO + 2SO_2$). Calcination: Heating carbonate/hydroxide ores in limited air (e.g., $ZnCO_3 \xrightarrow{\Delta} ZnO + CO_2$). (c) Reduction of Metallic Oxides: By Carbon/CO/Hydrogen: For less reactive metals (e.g., Cu, Pb, Fe, Zn). $CuO + C \xrightarrow{\Delta} Cu + CO$ $Fe_2O_3 + 3CO \xrightarrow{\Delta} 2Fe + 3CO_2$ By Electrolysis: For highly reactive metals (e.g., Na, K, Ca, Al). (d) Electrorefining: (Reference only, covered in Electrolysis). (iii) Extraction of Aluminium (a) Chemical Method for Purifying Bauxite (Bayer's Process): Bauxite ($Al_2O_3 \cdot 2H_2O$) is digested with concentrated NaOH to form soluble sodium aluminate. $Al_2O_3 \cdot 2H_2O + 2NaOH \rightarrow 2NaAlO_2 + 3H_2O$ Impurities (e.g., $Fe_2O_3$) are filtered out. Sodium aluminate is diluted and seeded with $Al(OH)_3$ to precipitate pure $Al(OH)_3$. $NaAlO_2 + 2H_2O \rightarrow Al(OH)_3 + NaOH$ $Al(OH)_3$ is heated to obtain pure alumina ($Al_2O_3$). $2Al(OH)_3 \xrightarrow{1000^\circ C} Al_2O_3 + 3H_2O$ (b) Electrolytic Extraction (Hall-Heroult's Process): Electrolyte: Molten alumina ($Al_2O_3$) dissolved in cryolite ($Na_3AlF_6$) and fluorspar ($CaF_2$). Cryolite lowers melting point and increases conductivity. Fluorspar lowers melting point and increases fluidity. Electrodes: Graphite rods as anode, carbon lining as cathode. Anode (oxidation): $2O^{2-} \rightarrow O_2(g) + 4e^-$; $C(s) + O_2(g) \rightarrow CO_2(g)$ (anode is consumed). Cathode (reduction): $Al^{3+} + 3e^- \rightarrow Al(l)$ (molten Al collects at bottom). (iv) Alloys (Composition & Uses) Stainless Steel: Fe, Cr, Ni (Utensils, surgical instruments) Duralumin: Al, Cu, Mg, Mn (Aircraft parts, pressure cookers) Brass: Cu, Zn (Utensils, decorative items) Bronze: Cu, Sn (Statues, medals) Fuse Metal/Solder: Pb, Sn (Soldering, electrical fuses) 8. Study of Compounds A. Hydrogen Chloride (HCl) Preparation: $NaCl(s) + H_2SO_4(conc.) \xrightarrow{ Density: Heavier than air. Solubility: Highly soluble in water (Fountain Experiment). Preparation of Hydrochloric Acid: Inverted funnel arrangement to prevent back-suction. Reactions: With Ammonia: $HCl(g) + NH_3(g) \rightarrow NH_4Cl(s)$ (Dense white fumes) Acidic properties: With metals: $Zn + 2HCl \rightarrow ZnCl_2 + H_2$ With oxides: $CuO + 2HCl \rightarrow CuCl_2 + H_2O$ With hydroxides: $NaOH + HCl \rightarrow NaCl + H_2O$ With carbonates: $Na_2CO_3 + 2HCl \rightarrow 2NaCl + H_2O + CO_2$ Precipitation reactions: $AgNO_3(aq) + HCl(aq) \rightarrow AgCl(s) + HNO_3(aq)$ (White ppt) $Pb(NO_3)_2(aq) + 2HCl(aq) \rightarrow PbCl_2(s) + 2HNO_3(aq)$ (White ppt, soluble in hot water) B. Ammonia ($NH_3$) Laboratory Preparation: $2NH_4Cl(s) + Ca(OH)_2(s) \xrightarrow{\Delta} CaCl_2(s) + 2H_2O(l) + 2NH_3(g)$ (Collected by downward displacement of air). From Nitrides: $Mg_3N_2 + 6H_2O \rightarrow 3Mg(OH)_2 + 2NH_3$ Haber's Process (Manufacture): $N_2(g) + 3H_2(g) \underset{Fe/Mo}{\overset{450-500^\circ C, 200atm}{\rightleftharpoons}} 2NH_3(g)$ Density: Lighter than air. Solubility: Highly soluble in water (Fountain Experiment). Reactions: With HCl: $NH_3(g) + HCl(g) \rightarrow NH_4Cl(s)$ (Dense white fumes) With hot Copper(II) Oxide: $3CuO(s) + 2NH_3(g) \xrightarrow{\Delta} 3Cu(s) + 3H_2O(l) + N_2(g)$ Burning in Oxygen: $4NH_3(g) + 3O_2(g) \xrightarrow{} 2N_2(g) + 6H_2O(l)$ (Greenish-yellow flame) Catalytic Oxidation: $4NH_3(g) + 5O_2(g) \xrightarrow{Pt/Rh, 800^\circ C} 4NO(g) + 6H_2O(g)$ With Chlorine: $NH_3$ (excess): $8NH_3 + 3Cl_2 \rightarrow N_2 + 6NH_4Cl$ $Cl_2$ (excess): $NH_3 + 3Cl_2 \rightarrow NCl_3 + 3HCl$ (Explosive, yellow liquid) Aqueous Ammonia ($NH_4OH$): With acids: $NH_4OH + HCl \rightarrow NH_4Cl + H_2O$ With metal salts (see Analytical Chemistry section for precipitates). Uses: Fertilizers, explosives, nitric acid, refrigerant. C. Nitric Acid ($HNO_3$) Laboratory Preparation: $KNO_3(s) + H_2SO_4(conc.) \xrightarrow{ Manufacture (Ostwald's Process): $4NH_3(g) + 5O_2(g) \xrightarrow{Pt/Rh, 800^\circ C} 4NO(g) + 6H_2O(g)$ $2NO(g) + O_2(g) \rightarrow 2NO_2(g)$ $3NO_2(g) + H_2O(l) \rightarrow 2HNO_3(aq) + NO(g)$ As Oxidising Agent: (Concentrated $HNO_3$) With Copper: $Cu + 4HNO_3(conc.) \rightarrow Cu(NO_3)_2 + 2NO_2 + 2H_2O$ With Carbon: $C + 4HNO_3(conc.) \rightarrow CO_2 + 4NO_2 + 2H_2O$ With Sulphur: $S + 6HNO_3(conc.) \rightarrow H_2SO_4 + 6NO_2 + 2H_2O$ D. Sulphuric Acid ($H_2SO_4$) Manufacture (Contact Process): $S(s) + O_2(g) \rightarrow SO_2(g)$ $2SO_2(g) + O_2(g) \xrightarrow{V_2O_5, 450^\circ C, 1-2atm} 2SO_3(g)$ $SO_3(g) + H_2SO_4(conc.) \rightarrow H_2S_2O_7(l)$ (Oleum) $H_2S_2O_7(l) + H_2O(l) \rightarrow 2H_2SO_4(aq)$ As Dilute Acid: With metals: $Mg + H_2SO_4(dil.) \rightarrow MgSO_4 + H_2$ With metal oxides: $CuO + H_2SO_4(dil.) \rightarrow CuSO_4 + H_2O$ With metal hydroxides: $2NaOH + H_2SO_4(dil.) \rightarrow Na_2SO_4 + 2H_2O$ With metal carbonates: $Na_2CO_3 + H_2SO_4(dil.) \rightarrow Na_2SO_4 + H_2O + CO_2$ As Concentrated Oxidising Agent: With Carbon: $C + 2H_2SO_4(conc.) \rightarrow CO_2 + 2SO_2 + 2H_2O$ With Sulphur: $S + 2H_2SO_4(conc.) \rightarrow 3SO_2 + 2H_2O$ As Dehydrating Agent: Dehydration of Sugar: $C_{12}H_{22}O_{11} \xrightarrow{H_2SO_4(conc.)} 12C + 11H_2O$ (Charring) Dehydration of $CuSO_4 \cdot 5H_2O$: $CuSO_4 \cdot 5H_2O \xrightarrow{H_2SO_4(conc.)} CuSO_4 + 5H_2O$ (Blue to white) Non-volatile nature: Used to prepare more volatile acids from their salts. $NaCl + H_2SO_4(conc.) \xrightarrow{ $KNO_3 + H_2SO_4(conc.) \xrightarrow{ 9. Organic Chemistry (i) Introduction to Organic Compounds Unique Nature of Carbon: Tetravalency: Forms four bonds. Catenation: Forms long chains, branched chains, and rings with other carbon atoms. Bonds: Single, double, triple bonds. Structures: Straight chain, branched chain, cyclic (e.g., Benzene). (ii) Structure and Isomerism Structural Formulae: Alkanes (e.g., Methane $CH_4$, Ethane $C_2H_6$, Propane $C_3H_8$, Butane $C_4H_{10}$, Pentane $C_5H_{12}$) Alkenes (e.g., Ethene $C_2H_4$, Propene $C_3H_6$, Butene $C_4H_8$, Pentene $C_5H_{10}$) Alkynes (e.g., Ethyne $C_2H_2$, Propyne $C_3H_4$, Butyne $C_4H_6$, Pentyne $C_5H_8$) Isomerism: Compounds with same molecular formula but different structural formulae. Chain Isomerism: Different carbon chain arrangements (e.g., n-butane and isobutane). Position Isomerism: Different positions of functional group or substituent (e.g., 1-chloropropane and 2-chloropropane). (iii) Homologous Series Series of organic compounds with same functional group, similar chemical properties, and successive members differ by a $CH_2$ group. Characteristics: Same general formula. Gradual change in physical properties (e.g., melting point, boiling point increase with molecular mass). Similar chemical properties. Can be prepared by general methods. Examples: Alkane ($C_nH_{2n+2}$), Alkene ($C_nH_{2n}$), Alkyne ($C_nH_{2n-2}$) series. (iv) Simple Nomenclature (IUPAC & Trivial) Longest chain rule, smallest number for functional groups. Functional groups: Double bond, triple bond, alcoholic (-OH), aldehydic (-CHO), carboxylic (-COOH). Examples: Ethanol, Ethanal, Ethanoic acid. (v) Hydrocarbons: Alkanes, Alkenes, Alkynes Alkanes: Saturated, single bonds. General formula $C_nH_{2n+2}$. Methane ($CH_4$), Ethane ($C_2H_6$). Preparation: From Sodium Ethanoate: $CH_3COONa + NaOH \xrightarrow{CaO, \Delta} CH_4 + Na_2CO_3$ From Iodomethane: $CH_3I + 2[H] \xrightarrow{Zn/HCl} CH_4 + HI$ Combustion: $CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$ Substitution with Chlorine: $CH_4 + Cl_2 \xrightarrow{hv} CH_3Cl + HCl$ Alkenes: Unsaturated, at least one double bond. General formula $C_nH_{2n}$. Ethene ($C_2H_4$). Preparation: Dehydrohalogenation of alkyl halides: $CH_3CH_2Br + KOH(alc.) \xrightarrow{\Delta} CH_2=CH_2 + KBr + H_2O$ Dehydration of alcohols: $CH_3CH_2OH \xrightarrow{conc. H_2SO_4, 170^\circ C} CH_2=CH_2 + H_2O$ Addition reactions (H$_2$, $Cl_2$, $Br_2$, $I_2$): $CH_2=CH_2 + H_2 \xrightarrow{Ni, \Delta} CH_3-CH_3$ Alkynes: Unsaturated, at least one triple bond. General formula $C_nH_{2n-2}$. Ethyne ($C_2H_2$). Preparation: From Calcium Carbide: $CaC_2 + 2H_2O \rightarrow Ca(OH)_2 + C_2H_2$ From 1,2-dibromoethane: $CH_2Br-CH_2Br + 2KOH(alc.) \xrightarrow{\Delta} CH\equiv CH + 2KBr + 2H_2O$ Addition reactions (H$_2$, $Cl_2$, $Br_2$, $I_2$): $CH\equiv CH + 2H_2 \xrightarrow{Ni, \Delta} CH_3-CH_3$ Uses: Methane (fuel), Ethane (fuel, feedstock), Ethene (polythene, ripening), Ethyne (welding, cutting). (vi) Alcohols: Ethanol Preparation: Hydrolysis of alkyl halide: $CH_3CH_2Cl + KOH(aq) \xrightarrow{\Delta} CH_3CH_2OH + KCl$ Properties: Physical: Colorless liquid, characteristic smell, soluble in water, less dense than water, B.P. $78^\circ C$. Combustion: $CH_3CH_2OH + 3O_2 \rightarrow 2CO_2 + 3H_2O$ Action with Sodium: $2CH_3CH_2OH + 2Na \rightarrow 2CH_3CH_2ONa + H_2$ (Sodium ethoxide) Esterification with Acetic Acid: $CH_3COOH + CH_3CH_2OH \xrightarrow{conc. H_2SO_4, \Delta} CH_3COOCH_2CH_3 + H_2O$ (Ethyl acetate, fruity smell) Dehydration to Ethene: $CH_3CH_2OH \xrightarrow{conc. H_2SO_4, 170^\circ C} CH_2=CH_2 + H_2O$ Denatured Alcohol: Ethanol made unfit for drinking by adding poisonous substances (e.g., methanol, pyridine). Spurious Alcohol: Illegally produced alcohol, often containing methanol, which is highly toxic. Uses: Solvent, fuel, alcoholic beverages, antiseptic. (vii) Carboxylic Acids: Acetic Acid Structure: $CH_3COOH$ Properties: Physical: Pungent vinegar smell. Glacial acetic acid (pure acetic acid) freezes below $17^\circ C$ to ice-like crystals. Chemical: Action with Litmus: Turns blue litmus red (weak acid). Action with Alkalis: $CH_3COOH + NaOH \rightarrow CH_3COONa + H_2O$ Esterification with Alcohol: $CH_3COOH + CH_3CH_2OH \xrightarrow{conc. H_2SO_4, \Delta} CH_3COOCH_2CH_3 + H_2O$ Uses: Vinegar (5-8% solution), solvent, coagulant for latex, in making plastics, dyes.