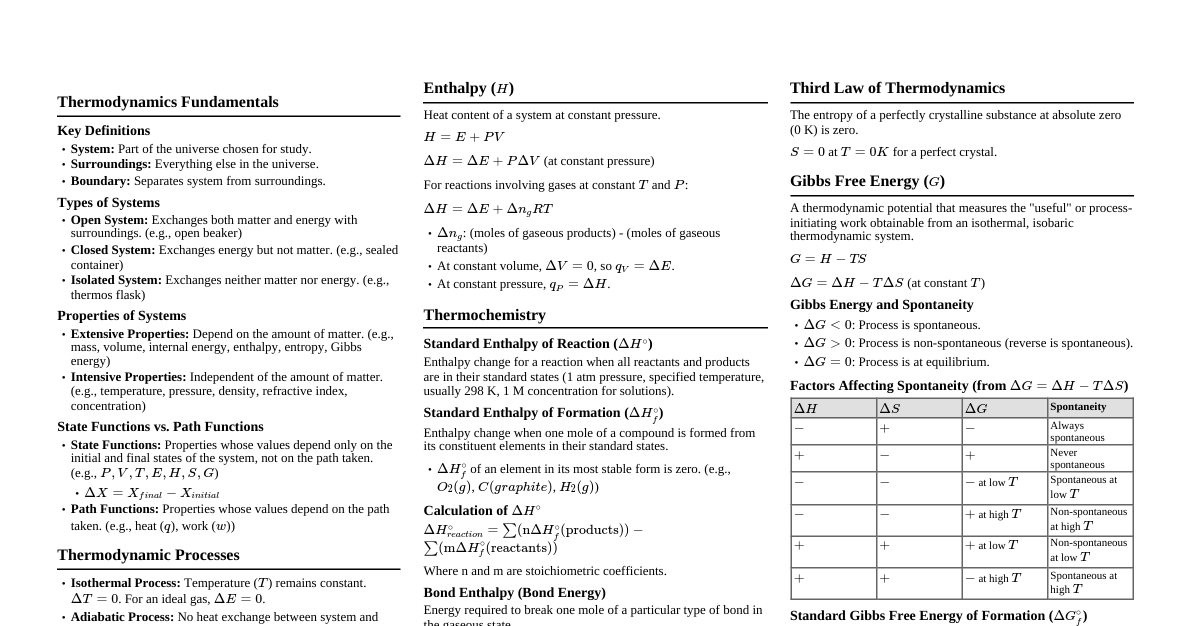
1. First Law of Thermodynamics Closed System: $dU = \delta Q + \delta W$ or $\Delta U = Q + W$ Open System (Steady State): $\Delta H + \Delta KE + \Delta PE = Q + W_s$ Kinetic Energy Change: $\Delta KE = \frac{1}{2} m (v_2^2 - v_1^2)$ Potential Energy Change: $\Delta PE = m g (z_2 - z_1)$ 2. Work and Heat Reversible P-V Work: $W = -\int P dV$ Shaft Work (Open System): $W_s = -\int V dP$ (for reversible process, negligible KE/PE) Heat Capacity at Constant Volume: $C_V = \left(\frac{\partial U}{\partial T}\right)_V$ Heat Capacity at Constant Pressure: $C_P = \left(\frac{\partial H}{\partial T}\right)_P$ Relationship for Ideal Gas: $C_P - C_V = R$ 3. Energy, Enthalpy Internal Energy (U): A state function representing microscopic energy. Enthalpy (H): $H = U + PV$ Specific Properties: $u = U/m$, $h = H/m$, etc. 4. Phase Rule Gibbs Phase Rule: $F = C - P + 2$ $F$: Degrees of freedom $C$: Number of components $P$: Number of phases 5. Volumetric Properties: PVT Behavior 5.1 Ideal Gas Equation of State: $PV = nRT$ or $P\underline{V} = RT$ Compressibility Factor: $Z = 1$ 5.2 Real Gas - Compressibility Factor Definition: $Z = \frac{P\underline{V}}{RT}$ 5.3 Cubic Equations of State General Form: $P = \frac{RT}{\underline{V}-b} - \frac{a}{\underline{V}^2 + k b \underline{V} + \epsilon b^2}$ Van der Waals (VDW): $P = \frac{RT}{\underline{V}-b} - \frac{a}{\underline{V}^2}$ $a = \frac{27 R^2 T_c^2}{64 P_c}$ $b = \frac{R T_c}{8 P_c}$ Redlich-Kwong (RK): $P = \frac{RT}{\underline{V}-b} - \frac{a}{T^{1/2}\underline{V}(\underline{V}+b)}$ $a = \frac{0.42748 R^2 T_c^{2.5}}{P_c}$ $b = \frac{0.08664 R T_c}{P_c}$ Soave-Redlich-Kwong (SRK): $P = \frac{RT}{\underline{V}-b} - \frac{a\alpha(T_r,\omega)}{\underline{V}(\underline{V}+b)}$ $a = \frac{0.42748 R^2 T_c^2}{P_c}$ $b = \frac{0.08664 R T_c}{P_c}$ $\alpha(T_r, \omega) = [1 + m(1 - T_r^{0.5})]^2$ $m = 0.480 + 1.574\omega - 0.176\omega^2$ Peng-Robinson (PR): $P = \frac{RT}{\underline{V}-b} - \frac{a\alpha(T_r,\omega)}{\underline{V}(\underline{V}+b)+b(\underline{V}-b)}$ $a = \frac{0.45724 R^2 T_c^2}{P_c}$ $b = \frac{0.07780 R T_c}{P_c}$ $\alpha(T_r, \omega) = [1 + m(1 - T_r^{0.5})]^2$ $m = 0.37464 + 1.54226\omega - 0.26992\omega^2$ 5.4 Virial Equation of State In terms of volume: $Z = 1 + \frac{B}{\underline{V}} + \frac{C}{\underline{V}^2} + \dots$ In terms of pressure: $Z = 1 + B'P + C'P^2 + \dots$ Relationship: $B' = B/RT$, $C' = (C-B^2)/(RT)^2$ 5.5 Generalized Correlations (Pitzer's Correlation) Compressibility Factor: $Z = Z^0 + \omega Z^1$ Reduced Temperature: $T_r = T/T_c$ Reduced Pressure: $P_r = P/P_c$ Acentric Factor ($\omega$): $\omega = - \log_{10}(P_{sat}/P_c)_{T_r=0.7} - 1.000$ 6. Second Law of Thermodynamics and Entropy 6.1 Statements Kelvin-Planck Statement: It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work. Clausius Statement: It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher-temperature body. 6.2 Entropy Entropy Change (Reversible Process): $dS = \frac{\delta Q_{rev}}{T}$ Entropy Change (Any Process): $dS \ge \frac{\delta Q}{T}$ (Equality for reversible, inequality for irreversible) Entropy Generation: $S_{gen} = \Delta S_{system} + \Delta S_{surroundings} \ge 0$ Entropy Change for Ideal Gas: $\Delta S = C_V \ln\left(\frac{T_2}{T_1}\right) + R \ln\left(\frac{V_2}{V_1}\right)$ $\Delta S = C_P \ln\left(\frac{T_2}{T_1}\right) - R \ln\left(\frac{P_2}{P_1}\right)$ Isentropic Process (Reversible Adiabatic): $\Delta S = 0$ 6.3 Heat Engines and Efficiency Thermal Efficiency of Heat Engine: $\eta_{th} = \frac{W_{net}}{Q_H} = 1 - \frac{Q_L}{Q_H}$ Carnot Efficiency: $\eta_{Carnot} = 1 - \frac{T_L}{T_H}$ Coefficient of Performance (COP) - Refrigerator: $COP_R = \frac{Q_L}{W_{net}} = \frac{1}{Q_H/Q_L - 1}$ COP - Heat Pump: $COP_{HP} = \frac{Q_H}{W_{net}} = \frac{1}{1 - Q_L/Q_H}$ Relationship between COPs: $COP_{HP} = COP_R + 1$ Carnot COP (Refrigerator): $COP_{R,Carnot} = \frac{T_L}{T_H - T_L}$ Carnot COP (Heat Pump): $COP_{HP,Carnot} = \frac{T_H}{T_H - T_L}$ 6.4 Thermodynamic Temperature Scale Definition: $\frac{Q_H}{Q_L} = \frac{T_H}{T_L}$ for a reversible heat engine. 7. Properties of Pure Substances Quality (x) - for Saturated Mixture: $x = \frac{m_{vapor}}{m_{total}}$ Specific Volume: $v = v_f + x(v_g - v_f) = v_f + x v_{fg}$ Specific Internal Energy: $u = u_f + x(u_g - u_f) = u_f + x u_{fg}$ Specific Enthalpy: $h = h_f + x(h_g - h_f) = h_f + x h_{fg}$ Specific Entropy: $s = s_f + x(s_g - s_f) = s_f + x s_{fg}$ Steam Tables and Mollier Diagram: Provide $P, T, v, u, h, s$ values for water/steam at various states. Values are read directly from tables/charts based on two independent properties.