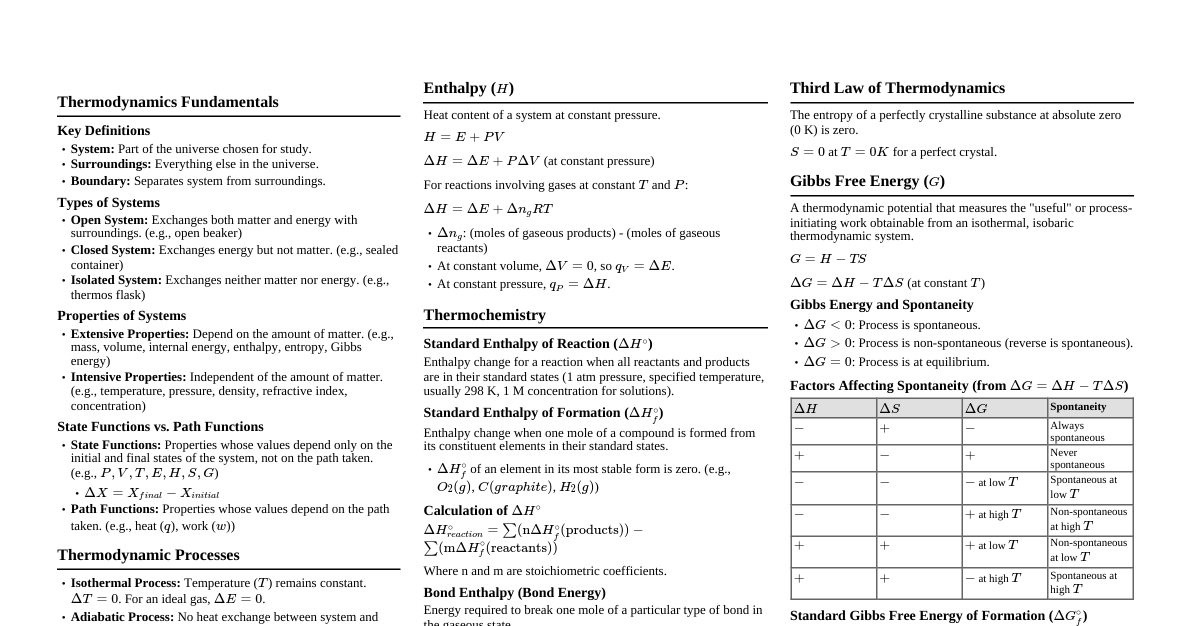
1. Terms in Thermodynamics 1.1. System and Surrounding System: Specified portion of the universe under study. Surrounding: Portion of the universe except the system. Open system: Both energy and matter exchange with surroundings. Example: Hot water in an open vessel. Closed system: Energy exchanges, but matter does not. Example: Hot water in a closed vessel. Isolated system: Neither energy nor matter exchanges. Example: Hot water in a thermal flask. 1.2. Process Isothermal process: Constant temperature ($dT=0$). Isobaric process: Constant pressure ($dP=0$). Isochoric process: Constant volume ($dV=0$). Adiabatic process: No heat exchange ($dq=0$). 1.3. State Function and Path Function State function: Thermodynamic variables depending only on initial and final states. Examples: Temperature, pressure, enthalpy, entropy, Gibbs' free energy, internal energy. Path function: Thermodynamic variables depending on the path followed. Examples: Heat, work. 1.4. Intensive and Extensive Property Intensive property: Independent of the amount of substance. Examples: Density, viscosity, surface tension, pressure, temperature, molar volume, molar heat capacity. Extensive property: Dependent on the amount of substance. Examples: Enthalpy, entropy, Gibbs' free energy, internal energy, mass, volume, heat capacity. 2. Internal Energy Sum of all energies possessed by a molecule in a system. Heat content at constant volume. Change in internal energy measured by Bomb Calorimeter. First Law of Thermodynamics Energy can neither be created nor be destroyed. Mathematical expression: $\Delta U = q + w$ $\Delta U$: Internal energy change $q$: Heat (positive if added to system, negative if liberated) $w$: Work done (positive if done on system, negative if done by system) Work done: $W = -P\Delta V$ 2.1. Work done in an Isothermal process $W = -2.303 nRT \log \frac{V_2}{V_1}$ 3. Enthalpy (H) Heat content of the system: $H = U + PV$ Change in enthalpy: $\Delta H = \Delta U + P\Delta V$ Significance: Heat absorbed or liberated by a system at constant pressure. For solids: $\Delta H = \Delta U$ 3.1. Relationship between $\Delta H$ and $\Delta U$ for gases $\Delta H = \Delta U + \Delta n_g RT$ $\Delta U$: Change in internal energy $\Delta n_g$: Change in number of moles of gaseous products and reactants $R$: Universal gas constant $T$: Temperature When $\Delta n_g = 0$, then $\Delta H = \Delta U$ (e.g., $H_{2(g)} + I_{2(g)} \rightarrow 2HI_{(g)}$) 3.2. Exothermic and Endothermic Reaction Exothermic reaction: Heat energy is liberated. $\Delta H$ is negative, $H_{Reactant} > H_{Product}$. Example: $C_{(s)} + O_{2(g)} \rightarrow CO_{2(g)} \quad \Delta H = -393.5 \text{ KJ}$ Endothermic reaction: Heat energy is absorbed. $\Delta H$ is positive, $H_{Product} > H_{Reactant}$. Example: $N_{2(g)} + 2O_{2(g)} \rightarrow 2NO_{2(g)} \quad \Delta H = +33.2 \text{ KJ}$ 3.3. Different types of enthalpies Standard enthalpy of formation ($\Delta_f H^\circ$): Enthalpy change when one mole of a compound is formed from its constituent elements in their standard states. Example: $C_{(s)} + O_{2(g)} \rightarrow CO_{2(g)} \quad \Delta H^\circ = -393.5 \text{ KJ}$ Standard enthalpy of combustion ($\Delta_c H^\circ$): Enthalpy change when one mole of a substance is burnt in excess air/oxygen in standard state. Example: $CH_{4(g)} + 2O_{2(g)} \rightarrow CO_{2(g)} + 2H_2O_{(l)} \quad \Delta H^\circ = -890.5 \text{ KJ}$ Always negative. Hydrocarbons act as fuel. Standard enthalpy of fusion ($\Delta_{fus} H^\circ$): Enthalpy change when one mole of solid converts to liquid at melting point. Example: $H_2O_{(s)} \rightarrow H_2O_{(l)} \quad \Delta_{fus} H^\circ = +6 \text{ KJ}$ Standard enthalpy of vaporization ($\Delta_{vap} H^\circ$): Enthalpy change when one mole of liquid converts to gaseous state at boiling point. Example: $H_2O_{(l)} \rightarrow H_2O_{(g)} \quad \Delta_{vap} H^\circ = +40 \text{ KJ}$ Standard enthalpy of sublimation ($\Delta_{sub} H^\circ$): Enthalpy change when one mole of solid directly converts to gaseous state below melting point. Example: $I_{2(s)} \rightarrow I_{2(g)} \quad \Delta_{sub} H^\circ = +73 \text{ KJ}$ Standard enthalpy of atomization ($\Delta_a H^\circ$): Enthalpy change when one molecule is converted to its atoms in standard condition. Example: $H_{2(g)} \rightarrow 2H_{(g)} \quad \Delta H^\circ = +435 \text{ KJ}$ Standard enthalpy of reaction ($\Delta H^\circ$): Enthalpy change when reactants are converted to product in their standard state. $\Delta H^\circ = \sum H_P - \sum H_R$ (from formation enthalpies) $\Delta H^\circ = \sum \text{Bond Enthalpy}_R - \sum \text{Bond Enthalpy}_P$ (from bond enthalpies) 3.4. Hess's Law The enthalpy change of a reaction is the same whether it takes place in one step or several steps. Illustration (formation of CO 2 ): Method 1: $C_{(s)} + O_{2(g)} \rightarrow CO_{2(g)} \quad \Delta H = -393.5 \text{ KJ}$ Method 2: $C_{(s)} + \frac{1}{2}O_{2(g)} \rightarrow CO_{(g)} \quad \Delta H_1 = -110.5 \text{ KJ}$ $CO_{(g)} + \frac{1}{2}O_{2(g)} \rightarrow CO_{2(g)} \quad \Delta H_2 = -283 \text{ KJ}$ $\Delta H = \Delta H_1 + \Delta H_2$ 3.5. Born-Haber Cycle Used to calculate lattice enthalpy of an ionic compound based on Hess's law. Lattice enthalpy: Enthalpy change when one mole of an ionic compound dissociates into its ions in gaseous state. For NaF: $\Delta_f H^\circ = \Delta_{sub} H^\circ + \frac{1}{2} \Delta_{Diss} H^\circ + IE + EA + \Delta_{lattice} H^\circ$ 4. Entropy Definition: Disorder of a system. $\Delta S = \frac{q_{rev}}{T}$ For a spontaneous reaction, $\Delta S$ should be positive. If $\Delta S$ is negative, the reaction is non-spontaneous. If $\Delta S = 0$, the reaction is at equilibrium. Predict the sign of $\Delta S$: Melting of ice: Positive Condensation of water vapor: Negative Sublimation of iodine: Positive Evaporation of water: Positive Dissolution of sugar in water: Positive Graphite to diamond: Negative Solid at $100K \rightarrow$ Solid at $0K$: Negative 4.1. Second Law of Thermodynamics The entropy of the universe increases in the course of every natural process. $\Delta S_{universe} > 0$ $\Delta S_{system} + \Delta S_{surrounding} > 0$ 4.2. Third Law of Thermodynamics The entropy of a perfectly crystalline solid is zero at absolute zero. 5. Gibbs' Free Energy Definition: Useful work obtained from a system. For a spontaneous reaction, $\Delta G$ should be negative. If $\Delta G$ is positive, the reaction is non-spontaneous. If $\Delta G = 0$, at equilibrium. 5.1. Relationship between $\Delta G$, $\Delta H$ and $\Delta S$ (Gibbs-Helmholtz Equation) $\Delta G = \Delta H - T\Delta S$ $\Delta G$: Change in Gibbs' free energy $\Delta H$: Change in enthalpy $\Delta S$: Change in entropy $T$: Temperature 5.2. Relationship between Gibbs' free energy change and Equilibrium constant $\Delta G = -2.303 RT \log K_c$ $\Delta G$: Change in Gibbs' free energy $R$: Universal gas constant $T$: Temperature $K_c$: Equilibrium constant 5.3. Criteria for predicting spontaneity of a process $\Delta H$ $\Delta S$ Spontaneity -ve +ve Spontaneous at all temperatures +ve -ve Non-spontaneous at all temperatures -ve -ve Spontaneous at low temperatures +ve +ve Spontaneous at high temperatures