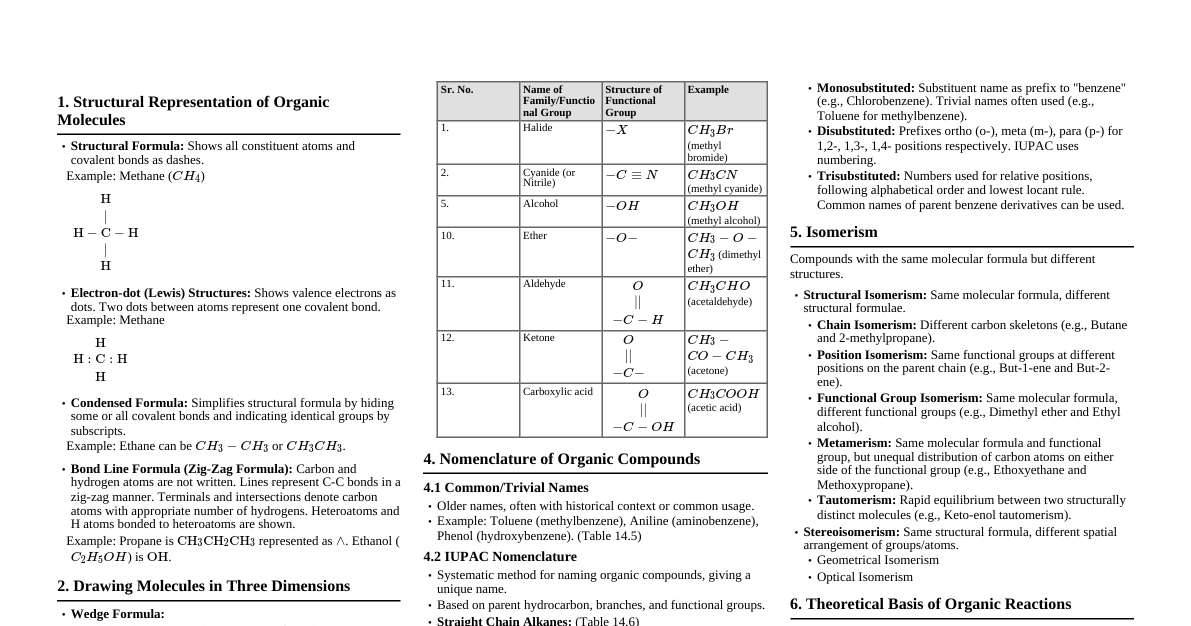
### Hydrocarbons: Introduction - **Definition:** Organic compounds composed solely of hydrogen and carbon atoms. - **Classification:** - **Aliphatic:** - **Saturated:** Alkanes (single bonds) - **Unsaturated:** Alkenes (double bonds), Alkynes (triple bonds) - **Aromatic:** Benzene and its derivatives (cyclic, conjugated, planar, 4n+2 $\pi$ electrons) ### Alkanes - **Formula:** $C_nH_{2n+2}$ - **Structure:** Saturated, open-chain hydrocarbons. $sp^3$ hybridization. - **Isomerism:** Chain isomerism (different carbon skeletons). - **Physical Properties:** - Nonpolar, immiscible with water. - Boiling point increases with molecular weight (due to increased van der Waals forces). - Branching decreases boiling point (less surface area for interaction). - Gaseous ($C_1-C_4$), liquid ($C_5-C_{17}$), solid ($>C_{18}$) at room temp. - **Chemical Properties (Reactions):** - **Halogenation (Free Radical Substitution):** - $CH_4 + Cl_2 \xrightarrow{hv} CH_3Cl + HCl$ - **Mechanism:** Initiation (homolytic cleavage of $Cl_2$), Propagation (chain reaction), Termination. - **Reactivity Order:** $3^\circ > 2^\circ > 1^\circ$ H atom. - **Selectivity:** Br is more selective than Cl. - **Combustion:** $C_nH_{2n+2} + (\frac{3n+1}{2})O_2 \rightarrow nCO_2 + (n+1)H_2O + \text{Energy}$ - **Pyrolysis/Cracking:** Decomposition at high temperatures to smaller hydrocarbons. ### Alkenes - **Formula:** $C_nH_{2n}$ - **Structure:** Unsaturated, contains at least one C=C double bond. $sp^2$ hybridization. - **Isomerism:** - Chain and positional isomerism. - **Geometric (cis-trans) isomerism:** Due to restricted rotation around C=C bond (if two different groups on each carbon of the double bond). - **Physical Properties:** Similar to alkanes, slightly lower boiling points than corresponding alkanes. - **Methods of Preparation:** - **Dehydration of Alcohols:** $R-CH_2-CH_2-OH \xrightarrow{Conc. H_2SO_4, \Delta} R-CH=CH_2 + H_2O$ (Saytzeff's Rule: more substituted alkene is major product). - **Dehydrohalogenation of Haloalkanes ($\beta$-elimination):** $R-CH_2-CH_2-X \xrightarrow{alc. KOH, \Delta} R-CH=CH_2 + HX$ (Saytzeff's Rule). - **Dehalogenation of Vicinal Dihalides:** $R-CHBr-CHBr-R' \xrightarrow{Zn, \text{alcohol}} R-CH=CH-R' + ZnBr_2$. #### Alkene Reactions - **Electrophilic Addition (Characteristic Reaction):** - **Addition of $H_2$ (Hydrogenation):** $C=C + H_2 \xrightarrow{Ni, Pt \text{ or } Pd} C-C$ (syn addition). - **Addition of Halogens ($X_2$):** $C=C + Br_2 \rightarrow \text{vicinal dibromide}$ (anti addition, via bromonium ion). - **Addition of $HX$ (Hydrohalogenation):** $C=C + HX \rightarrow \text{haloalkane}$ (Markovnikov's Rule: H adds to carbon with more H's; X adds to carbon with fewer H's). - **Peroxide Effect (Anti-Markovnikov):** Only with HBr in presence of peroxides (free radical mechanism). - **Addition of $H_2O$ (Hydration):** $C=C + H_2O \xrightarrow{H^+} \text{alcohol}$ (Markovnikov's Rule). - **Oxidation Reactions:** - **Baeyer's Reagent (Cold, Dilute, Alkaline $KMnO_4$):** $C=C \rightarrow \text{vicinal diol}$ (syn addition). - **Ozonolysis:** $C=C \xrightarrow{O_3, \text{then } Zn/H_2O} \text{aldehydes/ketones}$ (cleavage of double bond). ### Alkynes - **Formula:** $C_nH_{2n-2}$ - **Structure:** Unsaturated, contains at least one C$\equiv$C triple bond. $sp$ hybridization. - **Acidity of Terminal Alkynes:** Terminal alkynes have acidic H atoms ($pKa \approx 25$) due to high $s$-character of $sp$ orbital, allowing formation of acetylides. - $R-C\equiv C-H + NaNH_2 \rightarrow R-C\equiv C^- Na^+ + NH_3$ - **Physical Properties:** Similar to alkanes/alkenes, slightly higher boiling points due to stronger dipole-dipole interactions. Acetylene is gas. - **Methods of Preparation:** - **Dehydrohalogenation of Vicinal/Geminal Dihalides:** Requires strong base (e.g., $NaNH_2$) and high temperatures. - $R-CH_2-CHX_2 \xrightarrow{2NaNH_2, \Delta} R-C\equiv C-H + 2NaX + 2NH_3$ - $R-CX_2-CH_2-R' \xrightarrow{2NaNH_2, \Delta} R-C\equiv C-R' + 2NaX + 2NH_3$ #### Alkyne Reactions - **Electrophilic Addition:** Similar to alkenes, but two molecules of reagent can add. - **Hydrogenation:** - $R-C\equiv C-R' + H_2 \xrightarrow{Ni, Pt \text{ or } Pd} R-CH_2-CH_2-R'$ (complete reduction to alkane). - $R-C\equiv C-R' + H_2 \xrightarrow{\text{Lindlar's Catalyst}} \text{cis-alkene}$ (partial reduction). - $R-C\equiv C-R' + Na/Li \text{ in } NH_3(liq) \rightarrow \text{trans-alkene}$ (partial reduction, Birch reduction). - **Addition of Halogens ($X_2$):** $R-C\equiv C-R' + 2X_2 \rightarrow \text{tetrahaloalkane}$. - **Addition of $HX$:** $R-C\equiv C-H + HX \xrightarrow{Markovnikov} R-CX=CH_2 \xrightarrow{HX} R-CX_2-CH_3$. - **Addition of $H_2O$ (Hydration):** $R-C\equiv C-H + H_2O \xrightarrow{HgSO_4, H_2SO_4} \text{ketone}$ (via enol intermediate, Markovnikov). Acetylene gives acetaldehyde. - **Oxidation:** Strong oxidizing agents cleave the triple bond (similar to alkenes). - **Reactions of Terminal Alkynes:** - **Formation of Alkylides:** With $NaNH_2$, Tollens' reagent ($AgNO_3/NH_3$), or Fehling's solution ($CuCl/NH_4OH$). Useful for distinguishing terminal from internal alkynes. ### Aromatic Hydrocarbons (Arenes) - **Definition:** Cyclic, planar, conjugated systems with $(4n+2)\pi$ electrons (Hückel's Rule). - **Benzene:** Prototype aromatic compound. $C_6H_6$. Resonance stabilized. - **Physical Properties:** Nonpolar, immiscible with water, higher boiling points than non-aromatic counterparts. - **Chemical Properties:** - **Electrophilic Aromatic Substitution (EAS):** Benzene undergoes substitution, not addition, to retain aromaticity. - **Halogenation:** $C_6H_6 + Br_2 \xrightarrow{FeBr_3} C_6H_5Br + HBr$ - **Nitration:** $C_6H_6 + HNO_3 \xrightarrow{Conc. H_2SO_4} C_6H_5NO_2 + H_2O$ - **Sulfonation:** $C_6H_6 + H_2SO_4 \xrightarrow{\Delta} C_6H_5SO_3H + H_2O$ - **Friedel-Crafts Alkylation:** $C_6H_6 + R-Cl \xrightarrow{AlCl_3} C_6H_5-R + HCl$ (can rearrange, polysubstitution). - **Friedel-Crafts Acylation:** $C_6H_6 + RCOCl \xrightarrow{AlCl_3} C_6H_5COR + HCl$ (no rearrangement, no polysubstitution). - **Effect of Substituents on EAS:** - **Activating Groups (ortho/para directors):** Electron-donating (e.g., $-OH, -NH_2, -CH_3, -OCH_3$). Increase reactivity. - **Deactivating Groups (meta directors):** Electron-withdrawing (e.g., $-NO_2, -COOH, -CHO, -CN$). Decrease reactivity. - **Halogens:** Deactivating but ortho/para directing (due to resonance effects outweighing inductive effects). ### Haloalkanes (Alkyl Halides) - **Formula:** $R-X$ (X = F, Cl, Br, I) - **Classification:** $1^\circ, 2^\circ, 3^\circ$ based on carbon bearing halogen. - **Physical Properties:** - Polar molecules (C-X bond). - Boiling points: $RI > RBr > RCl > RF$ (due to increasing size/polarizability). - Boiling points increase with molecular weight. - Slightly soluble in water, soluble in organic solvents. - **Methods of Preparation:** - **From Alcohols:** - $R-OH + HX \xrightarrow{ZnCl_2 \text{ (Lucas reagent for } 1^\circ, 2^\circ)} R-X + H_2O$ ($3^\circ > 2^\circ > 1^\circ$ reactivity for alcohols). - $R-OH + SOCl_2 \xrightarrow{\text{Pyridine}} R-Cl + SO_2 + HCl$ (Thionyl chloride, best method for $R-Cl$). - $R-OH + PCl_5 \rightarrow R-Cl + POCl_3 + HCl$ - $R-OH + PBr_3 \rightarrow R-Br + H_3PO_3$ - **From Alkenes:** - **Hydrohalogenation:** $C=C + HX \rightarrow R-X$ (Markovnikov). - **Halogenation:** $C=C + X_2 \rightarrow \text{vicinal dihalide}$. - **From Alkanes:** Free radical halogenation (less useful due to mixtures). - **Halogen Exchange (Finkelstein & Swartz):** - **Finkelstein:** $R-Cl/Br + NaI \xrightarrow{\text{Acetone}} R-I + NaCl/NaBr$ (for alkyl iodides). - **Swartz:** $R-Cl/Br + AgF/Hg_2F_2/CoF_2/SbF_3 \rightarrow R-F + \text{metal salts}$ (for alkyl fluorides). #### Haloalkane Reactions - **Nucleophilic Substitution Reactions ($S_N1$ and $S_N2$):** - **$S_N2$ (bimolecular):** One step, concerted, inversion of configuration. - **Order of Reactivity:** $CH_3X > 1^\circ > 2^\circ > 3^\circ$ (steric hindrance). - **Favored by:** Strong nucleophiles, polar aprotic solvents (DMSO, acetone). - **$S_N1$ (unimolecular):** Two steps, carbocation intermediate, racemization. - **Order of Reactivity:** $3^\circ > 2^\circ > 1^\circ$ (carbocation stability). - **Favored by:** Weak nucleophiles, polar protic solvents ($H_2O$, alcohols). - **Nucleophiles:** $OH^-, CN^-, NH_3, H_2O, RO^-, RCOO^-, I^-$, etc. - **Examples:** - $R-X + KOH(aq) \rightarrow R-OH + KX$ (alcohol). - $R-X + KCN \rightarrow R-CN + KX$ (nitrile). - $R-X + AgCN \rightarrow R-NC + AgX$ (isonitrile, ambident nucleophile). - $R-X + NaOR' \rightarrow R-O-R' + NaX$ (Williamson Ether Synthesis). - $R-X + NH_3 \rightarrow R-NH_2 + HX$ (amine). - **Elimination Reactions (E1 and E2):** - **E2 (bimolecular):** One step, concerted, anti-periplanar geometry. - **Order of Reactivity:** $3^\circ > 2^\circ > 1^\circ$ (alkene stability). - **Favored by:** Strong bases, high temperature, alcoholic solvents. - **Saytzeff's Rule:** Major product is the more substituted alkene. - **E1 (unimolecular):** Two steps, carbocation intermediate, rearrangement possible. - **Order of Reactivity:** $3^\circ > 2^\circ > 1^\circ$ (carbocation stability). - **Favored by:** Weak bases, high temperature, polar protic solvents. - **Competition:** High temp favors E, strong nucleophile/base favors $S_N2$/E2. - **Reaction with Metals:** - **Grignard Reagents:** $R-X + Mg \xrightarrow{\text{dry ether}} R-MgX$ (highly reactive nucleophile/base). - **Wurtz Reaction:** $2R-X + 2Na \xrightarrow{\text{dry ether}} R-R + 2NaX$ (for symmetrical alkanes). ### Haloarenes (Aryl Halides) - **Formula:** $Ar-X$ (Halogen directly attached to benzene ring). - **Physical Properties:** - Slightly polar, higher boiling points than corresponding alkyl halides due to larger size and $\pi$-electron system. - Immiscible with water. - **Methods of Preparation:** - **From Benzene (EAS - Halogenation):** $C_6H_6 + Cl_2 \xrightarrow{FeCl_3} C_6H_5Cl + HCl$ - **From Aniline (Sandmeyer/Gattermann):** - **Diazotization:** $Ar-NH_2 \xrightarrow{NaNO_2/HCl, 0-5^\circ C} Ar-N_2^+Cl^-$ - **Sandmeyer:** $Ar-N_2^+Cl^- \xrightarrow{CuCl/HCl \text{ or } CuBr/HBr} Ar-Cl/Br + N_2$ - **Gattermann:** $Ar-N_2^+Cl^- \xrightarrow{Cu/HCl \text{ or } Cu/HBr} Ar-Cl/Br + N_2$ - **From Phenol (limited):** $C_6H_5OH + PCl_5 \rightarrow C_6H_5Cl + POCl_3 + HCl$ (low yield). #### Haloarene Reactions - **Nucleophilic Substitution:** Extremely difficult due to: 1. Resonance stabilization of C-X bond (partial double bond character). 2. $sp^2$ hybridized carbon (stronger C-X bond). 3. Repulsion between nucleophile and electron-rich aromatic ring. - **Exceptions (Activated Nucleophilic Aromatic Substitution):** - **Electron-withdrawing groups (EWG) at ortho/para positions:** Stabilize the intermediate carbanion. - **Example:** Chlorobenzene with activating groups like $-NO_2$. - **Dow's Process:** $C_6H_5Cl \xrightarrow{NaOH, 623K, 300atm} C_6H_5ONa \xrightarrow{H^+} C_6H_5OH$ (Phenol formation). - **Electrophilic Aromatic Substitution (EAS):** Halogens are deactivating but ortho/para directing. - **Halogenation:** $C_6H_5Cl + Cl_2 \xrightarrow{FeCl_3} o/p-dichlorobenzene$ - **Nitration:** $C_6H_5Br + HNO_3 \xrightarrow{Conc. H_2SO_4} o/p-bromonitrobenzene$ - **Reaction with Metals:** - **Wurtz-Fittig Reaction:** $Ar-X + R-X + 2Na \xrightarrow{\text{dry ether}} Ar-R + 2NaX$ (for alkylated aromatics). - **Fittig Reaction:** $2Ar-X + 2Na \xrightarrow{\text{dry ether}} Ar-Ar + 2NaX$ (for biaryls).