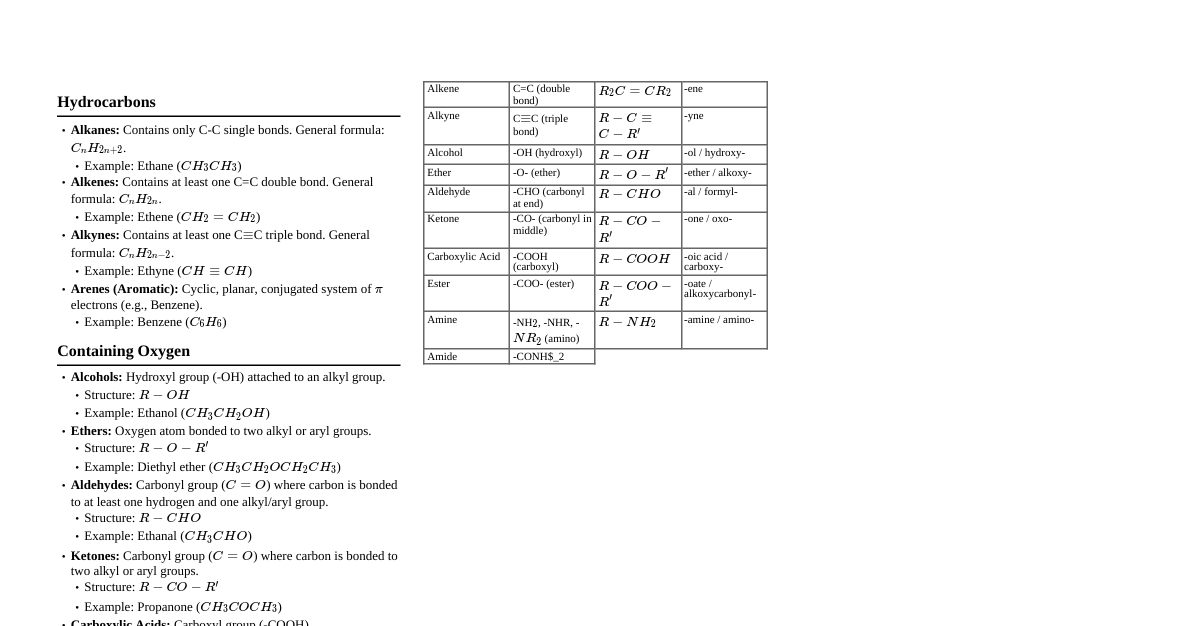
### Functional Groups - **Alkanes:** C-C single bonds (saturated) - **Alkenes:** C=C double bonds (unsaturated) - **Alkynes:** C≡C triple bonds - **Arenes:** Aromatic rings (e.g., Benzene) - **Alcohols:** R-OH (hydroxyl group) - **Ethers:** R-O-R' (ether linkage) - **Aldehydes:** R-CHO (carbonyl at end) - **Ketones:** R-CO-R' (carbonyl in middle) - **Carboxylic Acids:** R-COOH (carboxyl group) - **Esters:** R-COO-R' (ester linkage) - **Amines:** R-NH$_2$, R$_2$NH, R$_3$N (amino group) - **Amides:** R-CONH$_2$ (amide linkage) - **Nitriles:** R-C≡N (cyano group) - **Thiols:** R-SH (sulfhydryl group) ### S$_N$1, S$_N$2, E1, E2 Reactions #### S$_N$2 (Substitution, Nucleophilic, Bimolecular) - **Mechanism:** Concerted (one step), backside attack - **Substrate:** Primary > Secondary (Tertiary unreactive) - **Nucleophile:** Strong, unhindered - **Leaving Group:** Good (e.g., Cl, Br, I, TsO) - **Solvent:** Polar aprotic (e.g., DMSO, Acetone, DMF) - **Stereochemistry:** Inversion of configuration #### S$_N$1 (Substitution, Nucleophilic, Unimolecular) - **Mechanism:** Two steps, carbocation intermediate - **Substrate:** Tertiary > Secondary (Primary unreactive) - **Nucleophile:** Weak - **Leaving Group:** Good - **Solvent:** Polar protic (e.g., H$_2$O, EtOH, MeOH) - **Stereochemistry:** Racemization #### E2 (Elimination, Bimolecular) - **Mechanism:** Concerted, anti-periplanar transition state - **Substrate:** Primary, Secondary, Tertiary - **Base:** Strong, bulky preferred for Hofmann product - **Leaving Group:** Good - **Solvent:** Polar aprotic - **Product:** Zaitsev's rule (most substituted alkene) unless bulky base #### E1 (Elimination, Unimolecular) - **Mechanism:** Two steps, carbocation intermediate - **Substrate:** Tertiary > Secondary (Primary unreactive) - **Base:** Weak - **Leaving Group:** Good - **Solvent:** Polar protic - **Product:** Zaitsev's rule ### Alkene Reactions - **Hydrogenation:** R-CH=CH-R' + H$_2$ (Pd/Pt/Ni) → R-CH$_2$-CH$_2$-R' (Syn addition) - **Halogenation:** R-CH=CH-R' + X$_2$ (CCl$_4$) → R-CHX-CHX-R' (Anti addition) - **Hydrohalogenation:** R-CH=CH$_2$ + HX → R-CHX-CH$_3$ (Markovnikov) - **Hydrohalogenation (Peroxides):** R-CH=CH$_2$ + HBr (ROOR) → R-CH$_2$-CH$_2$-Br (Anti-Markovnikov) - **Hydration (Acid-catalyzed):** R-CH=CH$_2$ + H$_2$O (H$_2$SO$_4$) → R-CH(OH)-CH$_3$ (Markovnikov) - **Oxymercuration-Demercuration:** 1. Hg(OAc)$_2$, H$_2$O 2. NaBH$_4$ → R-CH(OH)-CH$_3$ (Markovnikov, Anti addition overall) - **Hydroboration-Oxidation:** 1. BH$_3 \cdot$ THF 2. H$_2$O$_2$, NaOH → R-CH$_2$-CH$_2$-OH (Anti-Markovnikov, Syn addition overall) - **Epoxidation:** R-CH=CH-R' + m-CPBA → Epoxide - **Dihydroxylation (Syn):** R-CH=CH-R' + OsO$_4$, H$_2$O$_2$ or KMnO$_4$ (cold, dilute, neutral) → Syn-diol - **Dihydroxylation (Anti):** R-CH=CH-R' + 1. m-CPBA 2. H$_3$O$^+$ → Anti-diol - **Ozonolysis:** 1. O$_3$ 2. DMS or Zn/H$_3$O$^+$ → Cleavage to aldehydes/ketones ### Carbonyl Chemistry (Aldehydes & Ketones) - **Nucleophilic Addition:** - **Hydride Reduction:** R-CHO/R-CO-R' + NaBH$_4$ or LiAlH$_4$ → Alcohol - **Grignard Reagent:** R-CHO/R-CO-R' + R'-MgX → Alcohol (with new C-C bond) - **Cyanohydrin Formation:** R-CHO/R-CO-R' + HCN → Cyanohydrin - **Imine Formation:** R-CHO/R-CO-R' + R'-NH$_2$ → Imine (R-CH=NR' or R$_2$C=NR') - **Enamine Formation:** R-CHO/R-CO-R' + R$_2$NH → Enamine - **Acetal/Ketal Formation:** R-CHO/R-CO-R' + ROH (acid catalyst) → Acetal/Ketal - **Wittig Reaction:** R-CHO/R-CO-R' + Ph$_3$P=CR$_2$ → Alkene (C=C formation) - **Aldol Condensation:** Enolate + Carbonyl → $\beta$-hydroxy carbonyl (then $\alpha,\beta$-unsaturated carbonyl) - **Cannizzaro Reaction:** Aldehydes without $\alpha$-hydrogens + OH$^-$ → Alcohol + Carboxylate ### Carboxylic Acid Derivatives - **Relative Reactivity:** Acid Chloride > Anhydride > Ester $\approx$ Carboxylic Acid > Amide - **Hydrolysis:** All derivatives can be hydrolyzed to carboxylic acids (acid or base catalyzed) - **Reduction (LiAlH$_4$):** - Carboxylic Acid → Primary Alcohol - Ester → Primary Alcohol - Amide → Amine - **Esterification (Fischer):** R-COOH + R'-OH (H$^+$) $\rightleftharpoons$ R-COO-R' + H$_2$O - **Amidation:** R-COOH + R'-NH$_2$ (heat) → R-CONH-R' - **Transesterification:** R-COO-R' + R''-OH $\rightleftharpoons$ R-COO-R'' + R'-OH ### Aromatic Reactions (Electrophilic Aromatic Substitution) - **General Mechanism:** Electrophile attacks $\pi$ system, followed by loss of H$^+$ - **Halogenation:** Benzene + X$_2$ (FeX$_3$) → Halobenzene - **Nitration:** Benzene + HNO$_3$ (H$_2$SO$_4$) → Nitrobenzene - **Sulfonation:** Benzene + SO$_3$ (H$_2$SO$_4$) → Benzenesulfonic Acid - **Friedel-Crafts Alkylation:** Benzene + R-X (AlCl$_3$) → Alkylbenzene (can rearrange, polysubstitution) - **Friedel-Crafts Acylation:** Benzene + R-COCl (AlCl$_3$) → Acylbenzene (no rearrangement, no polysubstitution) - **Activating Groups (ortho/para directors):** -OH, -OR, -NH$_2$, -NR$_2$, -R, -Ar - **Deactivating Groups (meta directors):** -NO$_2$, -CN, -COOH, -COOR, -SO$_3$H, -CHO, -COR - **Halogens:** Deactivating, but ortho/para directors ### Amine Reactions - **Basicity:** Aliphatic amines > Ammonia > Aromatic amines (electron-donating groups increase, electron-withdrawing decrease) - **Acylation:** R-NH$_2$ + R'-COCl (or anhydride) → Amide - **Hofmann Elimination:** R-CH$_2$-CH$_2$-NR$_3^+$ (strong base, heat) → Alkene (Hofmann product: least substituted) - **Diazotization (Primary Aromatic Amines):** Ar-NH$_2$ + NaNO$_2$, HCl (0-5°C) → Aryldiazonium salt (Ar-N$_2^+$Cl$^-$) - **Reactions of Diazonium Salts:** - **Sandmeyer:** CuCl/CuBr/CuCN → Ar-Cl/Ar-Br/Ar-CN - **Fluorination:** HBF$_4$, heat → Ar-F - **Iodination:** KI → Ar-I - **Hydrolysis:** H$_2$O, heat → Ar-OH - **Reduction:** H$_3$PO$_2$ → Ar-H - **Coupling:** With activated aromatic ring → Azo dye