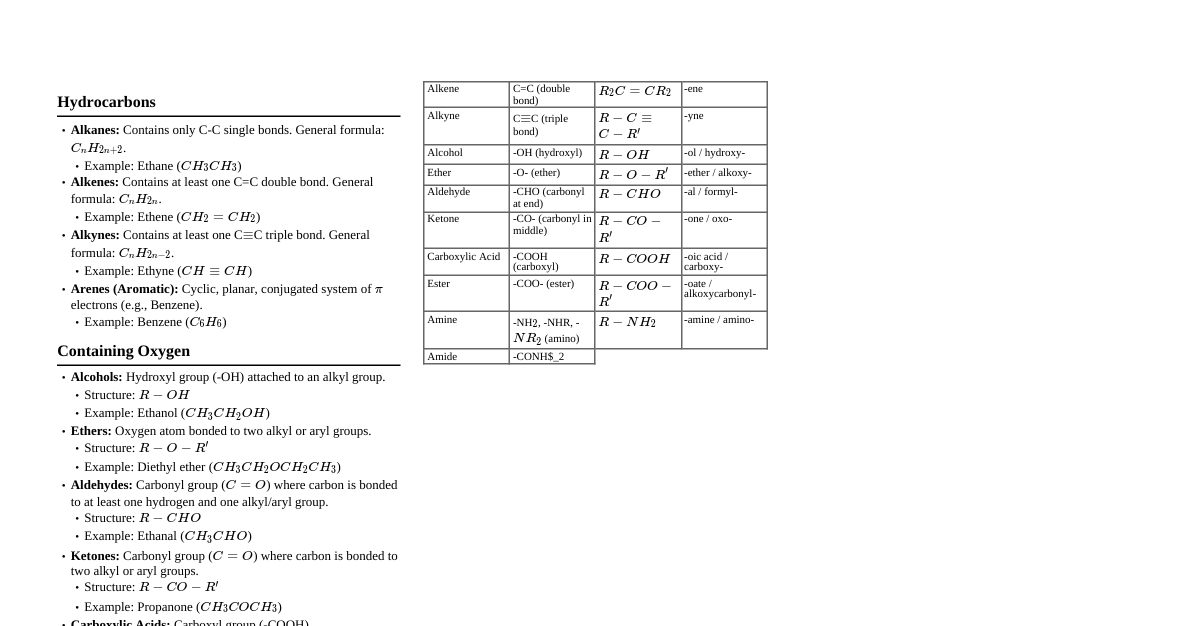
### 1. Basic Concepts & Nomenclature - **Hybridization:** - sp³: single bonds (tetrahedral, 109.5°) - sp²: one double bond (trigonal planar, 120°) - sp: one triple bond or two double bonds (linear, 180°) - **Inductive Effect (I-effect):** - Electron-donating (+I): Alkyl groups (CH₃ > CH₃CH₂ > etc.) - Electron-withdrawing (-I): -NO₂, -CN, -COOH, -Halogens - Decreases with distance. - **Resonance Effect (M-effect/R-effect):** - Delocalization of π-electrons or lone pairs. - Contributes to stability. - Electron-donating (+M): -OH, -OR, -NH₂, -X (halogens) - Electron-withdrawing (-M): -NO₂, -CN, -CHO, -COOH - **Hyperconjugation:** - Delocalization of σ-electrons (C-H bond) into an adjacent empty p-orbital or π-orbital. - "No bond resonance." More α-hydrogens = more stable carbocation/alkene. - **Nomenclature (IUPAC):** 1. Longest carbon chain (parent chain). 2. Number chain to give substituents lowest possible numbers. 3. Alphabetical order for multiple substituents. 4. Functional group priority: Carboxylic Acid > Sulfonic Acid > Ester > Acyl Halide > Amide > Nitrile > Aldehyde > Ketone > Alcohol > Amine > Alkene > Alkyne > Haloalkane > Alkane. ### 2. Isomerism - **Structural Isomerism:** Same molecular formula, different connectivity. - **Chain Isomerism:** Different carbon skeleton. - **Positional Isomerism:** Different position of functional group/substituent. - **Functional Isomerism:** Different functional groups. - **Metamerism:** Different alkyl groups attached to the same functional group (e.g., ethers, ketones, 2° amines). - **Tautomerism:** Rapid interconversion between two forms (e.g., keto-enol). Requires an α-hydrogen. - **Stereoisomerism:** Same connectivity, different spatial arrangement. - **Conformational Isomerism:** Interconvertible by rotation around C-C single bonds (e.g., Newman projections: staggered, eclipsed). - **Configurational Isomerism:** Not interconvertible without breaking bonds. - **Geometrical (cis-trans/E-Z):** Restricted rotation (e.g., alkenes, cyclic compounds). - **cis/trans:** Same groups on same/opposite sides. - **E/Z:** Priority rules (CIP) for complex alkenes. E = opposite, Z = same. - **Optical (Enantiomers/Diastereomers):** Presence of chiral center(s). - **Chiral Center:** Carbon atom bonded to four different groups. - **Enantiomers:** Non-superimposable mirror images. Rotate plane-polarized light in opposite directions. - **Diastereomers:** Stereoisomers that are not mirror images. - **Meso Compounds:** Possess chiral centers but are achiral due to internal plane of symmetry. - **Racemic Mixture:** 50:50 mixture of enantiomers, optically inactive. - **Specific Rotation:** $[\alpha]_D^T = \frac{\alpha}{l \times c}$ (where $\alpha$ = observed rotation, $l$ = path length, $c$ = concentration). ### 3. Reaction Mechanisms - **Electrophiles (E⁺):** Electron-deficient species (e.g., H⁺, NO₂⁺, Br⁺, Carbocations). - **Nucleophiles (Nu⁻):** Electron-rich species (e.g., OH⁻, CN⁻, NH₃, H₂O). - **Carbocations:** sp² hybridized, trigonal planar, electron-deficient. Stability: 3° > 2° > 1° > CH₃⁺. Undergo rearrangements (hydride/alkyl shifts). - **Carbanions:** sp³ hybridized (usually), pyramidal, electron-rich. Stability: CH₃⁻ > 1° > 2° > 3°. Stabilized by -I, -M groups. - **Free Radicals:** sp² hybridized (usually), planar, odd electron. Stability: 3° > 2° > 1° > CH₃•. - **Reaction Types:** - **Substitution:** One atom/group replaced by another. - **Nucleophilic Substitution (SN1/SN2):** - **SN1:** 2 steps, carbocation intermediate, 1st order, racemization, 3° > 2° > 1°. Favored by polar protic solvents. - **SN2:** 1 step, transition state, 2nd order, inversion of configuration, 1° > 2° > 3°. Favored by polar aprotic solvents. - **Electrophilic Substitution (SEAr):** Aromatic compounds (e.g., nitration, halogenation, Friedel-Crafts). - **Free Radical Substitution:** Alkanes (e.g., halogenation). - **Addition:** Atoms/groups added across a multiple bond (alkenes, alkynes). - **Electrophilic Addition:** Alkenes/Alkynes (e.g., HBr, H₂O). Follows Markovnikov's rule (H adds to C with more H's). Anti-Markovnikov with peroxides for HBr. - **Nucleophilic Addition:** Aldehydes/Ketones (e.g., HCN, Grignard reagents). - **Free Radical Addition:** Alkenes with HBr in presence of peroxides. - **Elimination (E1/E2):** Removal of atoms/groups to form a multiple bond. - **E1:** 2 steps, carbocation intermediate, 1st order, 3° > 2° > 1°. Favored by weak bases, polar protic solvents. - **E2:** 1 step, transition state, 2nd order, anti-periplanar geometry. Favored by strong bases, polar aprotic solvents. - Follows Saytzeff's rule (more substituted alkene is major product), Hofmann's rule (less substituted alkene) for bulky bases. - **Rearrangement:** Migration of atoms/groups within a molecule. ### 4. Hydrocarbons - **Alkanes:** - **Preparation:** Wurtz reaction, Decarboxylation, Hydrogenation of alkenes/alkynes, Kolbe's electrolytic method. - **Reactions:** Free radical halogenation, Combustion, Pyrolysis. - **Alkenes:** - **Preparation:** Dehydration of alcohols, Dehydrohalogenation of alkyl halides, Hofmann elimination, Wittig reaction, Partial hydrogenation of alkynes (Lindlar's catalyst for cis, Na/Li in NH₃ for trans). - **Reactions:** - **Electrophilic Addition:** HBr, H₂O (acid-catalyzed), HX, X₂ (anti-addition). - **Hydroboration-Oxidation:** (BH₃, H₂O₂, OH⁻) Anti-Markovnikov addition of H₂O (syn). - **Oxymercuration-Demercuration:** (Hg(OAc)₂, H₂O/NaBH₄) Markovnikov addition of H₂O (anti). - **Oxidation:** Baeyer's reagent (cold, dilute KMnO₄) → diols (syn). Hot KMnO₄ or Ozonolysis (O₃/H₂O or O₃/Zn, H₂O) → cleavage. - **Polymerization.** - **Alkynes:** - **Preparation:** Dehydrohalogenation of gem/vicinal dihalides, From calcium carbide. - **Reactions:** - **Electrophilic Addition:** HBr, H₂O (HgSO₄/H₂SO₄ → ketones/aldehydes). - **Acidity of Terminal Alkynes:** React with Na, NaNH₂, Grignard reagents. - **Hydrogenation:** (H₂/Pd/C, Lindlar's catalyst for cis-alkene; Na/NH₃ for trans-alkene). - **Polymerization.** - **Aromatic Hydrocarbons (Benzene):** - **Aromaticity:** Hückel's Rule (4n+2 π electrons), planar, cyclic, conjugated. - **Preparation:** Decarboxylation of benzoic acid, Phenol distillation with Zn dust, Cyclization of n-hexane. - **Electrophilic Aromatic Substitution (SEAr):** - **Nitration:** HNO₃/H₂SO₄ (NO₂⁺) - **Halogenation:** X₂/FeX₃ (X⁺) - **Sulfonation:** H₂SO₄/SO₃ (SO₃H⁺) - **Friedel-Crafts Alkylation:** R-X/AlCl₃ (R⁺) (Rearrangements possible, polyalkylation). - **Friedel-Crafts Acylation:** R-CO-X/AlCl₃ (R-CO⁺) (No rearrangements, no polyacylation). - **Directing Groups (for disubstituted benzene):** - **Ortho-para directors (+M/+I):** -OH, -OR, -NH₂, -R, -X (halogens are deactivating but o,p-directing). - **Meta directors (-M/-I):** -NO₂, -CN, -CHO, -COOH, -SO₃H. ### 5. Haloalkanes & Haloarenes - **Haloalkanes (Alkyl Halides):** - **Preparation:** From alcohols (HX/ZnCl₂, PCl₃, PCl₅, SOCl₂), From alkanes (free radical halogenation), From alkenes (HX, X₂). - **Reactions:** - **Nucleophilic Substitution (SN1/SN2):** See Section 3. - **Elimination (E1/E2):** See Section 3. - **Reaction with Metals:** Grignard reagent formation (R-Mg-X), Wurtz reaction. - **Reduction:** H₂/Pd, LiAlH₄. - **Haloarenes (Aryl Halides):** - **Preparation:** Halogenation of benzene (X₂/FeX₃), Sandmeyer reaction (from diazonium salts). - **Reactions:** Less reactive towards nucleophilic substitution than haloalkanes due to resonance stabilization and sp² hybridized carbon. - **Electrophilic Aromatic Substitution:** Halogens are o,p-directing but deactivating. - **Reaction with Metals:** Wurtz-Fittig reaction, Fittig reaction. - **Nucleophilic Substitution:** Requires extreme conditions (high T, P) or strong electron-withdrawing groups at o/p positions. ### 6. Alcohols, Phenols & Ethers - **Alcohols:** - **Preparation:** From alkenes (hydration, hydroboration-oxidation, oxymercuration-demercuration), From carbonyl compounds (reduction with LiAlH₄/NaBH₄, Grignard reagents), Hydrolysis of alkyl halides. - **Reactions:** - **Acidity:** Weaker than water. Acidity: 1° > 2° > 3°. - **Reaction with Na:** Forms alkoxides. - **Esterification:** With RCOOH/H⁺. - **Oxidation:** 1° alcohols → aldehydes → carboxylic acids. 2° alcohols → ketones. 3° alcohols are resistant. (Reagents: PCC, CrO₃, KMnO₄). - **Dehydration:** H₂SO₄/heat → alkenes (E1/E2). - **Reaction with HX/PX₃/SOCl₂:** Forms alkyl halides. - **Phenols:** - **Preparation:** From diazonium salts, From cumene, Dow's process (chlorobenzene hydrolysis), From benzene sulfonic acid. - **Reactions:** - **Acidity:** More acidic than alcohols (resonance stabilized phenoxide ion). React with Na, NaOH. - **Electrophilic Aromatic Substitution:** -OH is o,p-directing, activating. (Nitration, Halogenation, Sulfonation, Friedel-Crafts). - **Reimer-Tiemann Reaction:** Phenol + CHCl₃/NaOH → Salicylaldehyde. - **Kolbe's Reaction:** Phenol + CO₂/NaOH → Salicylic acid. - **Oxidation:** With K₂Cr₂O₇/H₂SO₄ → benzoquinone. - **Reaction with Zn dust:** → Benzene. - **Ethers:** - **Preparation:** Williamson Synthesis (R-X + R'-ONa), Dehydration of alcohols (H₂SO₄). - **Reactions:** - **Cleavage by HI/HBr:** Forms alkyl halides and alcohol (SN1/SN2). - **Peroxide formation:** Ethers form explosive peroxides in presence of air and light. ### 7. Aldehydes & Ketones - **Preparation:** - **From Alcohols:** Oxidation (PCC for aldehydes, CrO₃ for ketones). - **From Alkenes:** Ozonolysis. - **From Alkynes:** Hydration (HgSO₄/H₂SO₄). - **From Acyl Chlorides:** Rosenmund reduction (H₂/Pd-BaSO₄) → Aldehydes. - **From Nitriles:** Stephen reaction (SnCl₂/HCl) → Aldehydes. DIBAL-H → Aldehydes. Grignard reagents → Ketones. - **Friedel-Crafts Acylation:** For aromatic ketones. - **Gattermann-Koch reaction:** Benzene + CO/HCl/AlCl₃ → Benzaldehyde. - **Reactions:** - **Nucleophilic Addition:** Aldehydes are more reactive than ketones (steric hindrance, electronic effect). - **Addition of HCN:** → Cyanohydrins. - **Addition of NaHSO₃:** → Bisulfite addition product. - **Addition of Grignard Reagents:** → Alcohols. - **Addition of Alcohols:** → Hemiacetals/Acetals (aldehydes), Hemiketals/Ketals (ketones). - **Addition of Ammonia Derivatives:** (NH₂-Z) → Imines, Oximes, Hydrazones, Semicarbazones (condensation reactions). - **Reduction:** - **To Alcohols:** (NaBH₄, LiAlH₄, H₂/Ni). - **To Hydrocarbons:** Clemmensen reduction (Zn-Hg/HCl), Wolff-Kishner reduction (NH₂NH₂/KOH/ethylene glycol). - **Oxidation:** - **Aldehydes:** Easily oxidized to carboxylic acids (Tollens' reagent: Ag(NH₃)₂⁺; Fehlings' solution: Cu²⁺/OH⁻; Benedict's solution). - **Ketones:** Resist mild oxidation, require strong conditions (cleavage). - **Aldol Condensation:** Carbonyl compound with α-hydrogen. Forms β-hydroxy aldehyde/ketone, then α,β-unsaturated carbonyl. - **Cannizzaro Reaction:** Aldehydes without α-hydrogen. Disproportionation (one oxidized, one reduced). - **Haloform Reaction:** Methyl ketones (CH₃-CO-R) or alcohols that oxidize to methyl ketones (e.g., ethanol, 2-propanol). Forms haloform (CHX₃) and carboxylate. - **Electrophilic Substitution:** Aromatic aldehydes/ketones (e.g., benzaldehyde) are meta-directing, deactivating. ### 8. Carboxylic Acids & Derivatives - **Carboxylic Acids:** - **Preparation:** Oxidation of 1° alcohols/aldehydes, From Grignard reagents (CO₂/H₃O⁺), Hydrolysis of nitriles/esters. - **Reactions:** - **Acidity:** Stronger than phenols/alcohols. Acidity order: HCOOH > CH₃COOH. Electron-withdrawing groups increase acidity, electron-donating groups decrease. - **Salt Formation:** React with NaOH, NaHCO₃. - **Esterification:** With alcohols (acid-catalyzed). - **Reduction:** LiAlH₄ → 1° alcohols. - **Decarboxylation:** Soda-lime (NaOH/CaO) → alkanes. Kolbe's electrolytic method. - **HVZ Reaction (Hell-Volhard-Zelinsky):** R-CH₂-COOH + X₂/Red P → R-CH(X)-COOH. - **Derivatives:** (Acid chlorides, Anhydrides, Esters, Amides) - **Relative Reactivity:** Acid Chloride > Anhydride > Ester > Amide (towards nucleophilic acyl substitution). - **Preparation:** - **Acid Chlorides:** RCOOH + SOCl₂/PCl₃/PCl₅. - **Anhydrides:** RCOOH + RCOOH (heat). - **Esters:** RCOOH + R'OH (Fischer esterification). - **Amides:** RCOOH + NH₃ (heat). - **Hydrolysis:** All derivatives hydrolyze to carboxylic acids under acidic or basic conditions. - **Reduction:** LiAlH₄ reduces all to alcohols (amides to amines). ### 9. Amines - **Classification:** 1°, 2°, 3° amines based on number of alkyl groups on nitrogen. - **Preparation:** - **Reduction of Nitro compounds:** (Sn/HCl, Fe/HCl, H₂/Pd). - **Ammonolysis of Alkyl Halides:** (R-X + NH₃) → mixture of 1°, 2°, 3° amines and quaternary salt. - **Gabriel Phthalimide Synthesis:** For 1° amines only. - **Hoffmann Bromamide Degradation:** (R-CONH₂ + Br₂/KOH) → R-NH₂ (one carbon less). - **Reduction of Nitriles/Amides:** (LiAlH₄). - **Reactions:** - **Basicity:** Due to lone pair on N. Basicity order (gas phase): 3° > 2° > 1° > NH₃. (Aqueous phase - steric/solvation effects): 2° > 1° > 3° > NH₃ (for alkyl amines). Aromatic amines are less basic than aliphatic amines. - **Alkylation:** With R-X → 2°, 3° amines, quaternary salts. - **Acylation:** With R-CO-Cl/R-CO-O-CO-R → Amides. - **Carbylamine Reaction (Isocyanide Test):** 1° amines + CHCl₃/KOH → foul-smelling isocyanide. - **Reaction with Nitrous Acid (HNO₂, NaNO₂/HCl):** - **1° Aliphatic Amines:** → Diazonium salt (unstable) → N₂ + alcohol. - **1° Aromatic Amines:** → Arenediazonium salt (stable at 0-5°C). - **2° Amines (aliphatic/aromatic):** → N-nitrosoamines (yellow oily layer). - **3° Amines:** No reaction (aliphatic), or p-nitrosoaniline (aromatic). - **Hinsberg's Test:** Distinguishes 1°, 2°, 3° amines using benzene sulfonyl chloride. - **1° Amine:** Forms N-alkylbenzenesulfonamide, soluble in NaOH. - **2° Amine:** Forms N,N-dialkylbenzenesulfonamide, insoluble in NaOH. - **3° Amine:** No reaction. ### 10. Biomolecules - **Carbohydrates:** - **Monosaccharides:** Glucose, Fructose. - **Disaccharides:** Sucrose, Maltose, Lactose. - **Polysaccharides:** Starch, Cellulose, Glycogen. - **Reducing Sugars:** Possess free aldehyde/ketone group (e.g., glucose, fructose, maltose, lactose). Reduce Tollens'/Fehling's. Sucrose is non-reducing. - **Glucose Reactions:** Osazone formation, Oxidation, Reduction. - **Anomers, Epimers.** - **Proteins:** - **Amino Acids:** Building blocks. Zwitterionic form. Peptide bond formation. - **Classification:** Essential/Non-essential. Acidic/Basic/Neutral. - **Structure:** Primary (sequence), Secondary (α-helix, β-pleated sheet), Tertiary (3D folding), Quaternary (multiple subunits). - **Denaturation:** Loss of biological activity due to change in 3D structure. - **Vitamins:** A, B, C, D, E, K. Water-soluble (B, C) vs. Fat-soluble (A, D, E, K). - **Nucleic Acids:** DNA, RNA. Nucleotides (base + sugar + phosphate). - **DNA Bases:** A, T, G, C. Double helix. - **RNA Bases:** A, U, G, C. Single strand. ### 11. Polymers - **Classification:** Natural/Synthetic, Addition/Condensation, Thermoplastic/Thermosetting. - **Addition Polymers:** Formed by addition across double/triple bonds. - **Polythene:** Low density (LDPE), High density (HDPE). - **Teflon:** Polytetrafluoroethylene. - **PAN:** Polyacrylonitrile (Orlon). - **Polypropylene, PVC.** - **Natural Rubber:** Isoprene monomer. - **Condensation Polymers:** Formed by elimination of small molecules (H₂O, HCl). - **Nylon-6,6:** Hexamethylenediamine + Adipic acid. - **Nylon-6:** Caprolactam. - **Polyester (Dacron/Terylene):** Terephthalic acid + Ethylene glycol. - **Bakelite:** Phenol + Formaldehyde. - **Melamine-formaldehyde resin.** - **Copolymerization:** Two or more different monomers. - **Biodegradable Polymers:** PHBV, Nylon 2-Nylon 6. ### 12. Chemistry in Everyday Life - **Drugs:** Antacids, Antihistamines, Tranquilizers, Analgesics, Antibiotics, Antiseptics, Disinfectants, Antifertility drugs. - **Food Chemistry:** Antioxidants, Preservatives, Artificial sweetening agents, Edible colors. - **Cleansing Agents:** Soaps, Detergents.