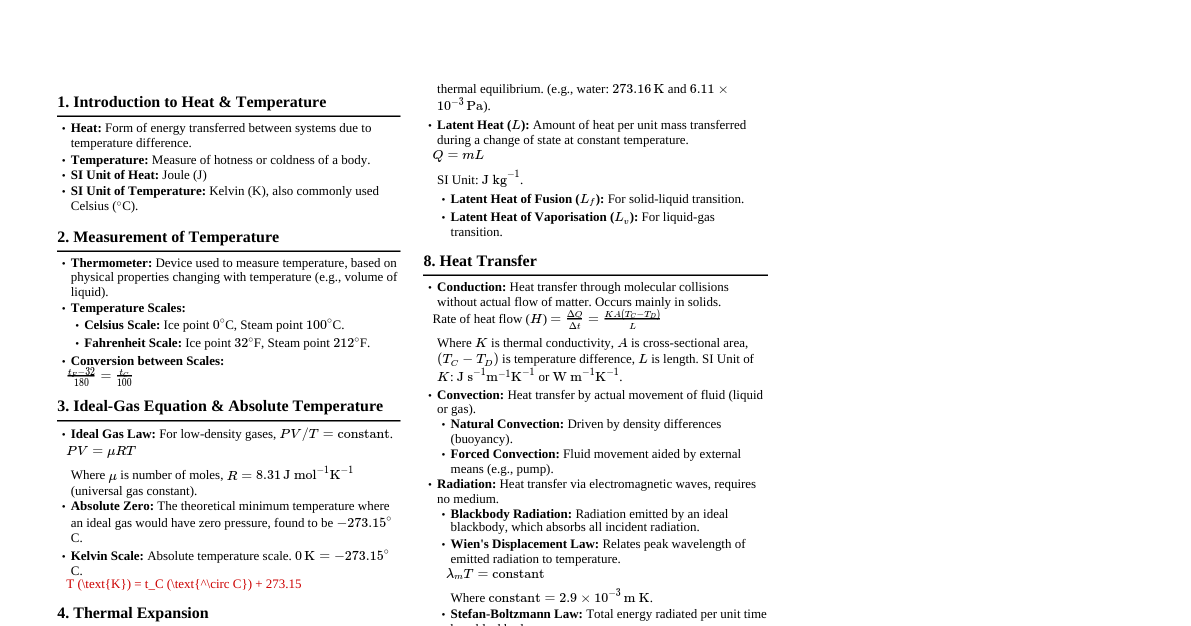
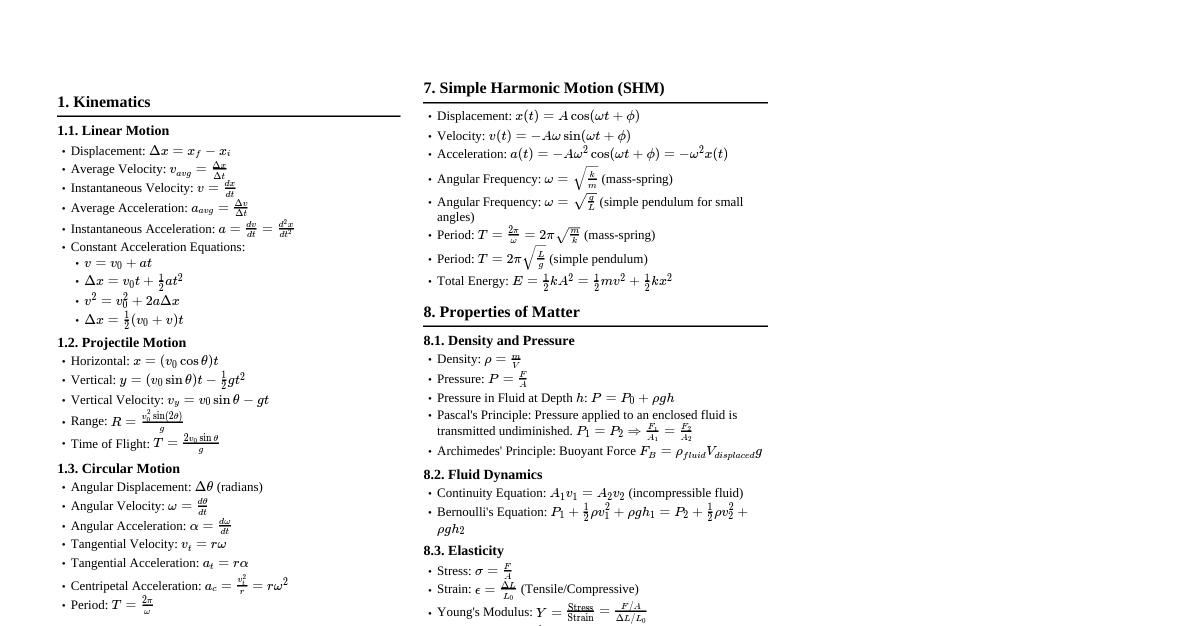
1. Introduction to Matter Definition: Matter is anything that possesses mass and occupies space (has volume). Early Philosophies: Ancient Indian Philosophers: Postulated "Panch Tatva" (air, earth, fire, sky, water) as the five basic elements from which all living and non-living things are made. Ancient Greek Philosophers: Also proposed similar ideas of fundamental elements. Modern Classification: Based on physical properties (covered in this cheatsheet). Based on chemical nature (elements, compounds, mixtures - covered in subsequent topics). 1.1 Physical Nature of Matter: Particulate Model 1.1.1 Matter is Made Up of Particles Debate: For centuries, scientists debated whether matter was continuous (like a block of wood) or particulate (made of tiny particles). Evidence: Dissolving Experiments: When substances like salt, sugar, or Dettol dissolve in water, their particles intermix with water particles without a significant change in total volume. This implies spaces between water particles. Example: Adding salt to water and stirring. The salt disappears, and the water level doesn't rise significantly, indicating salt particles filled the gaps between water particles. Conclusion: Matter is not continuous; it is composed of discrete, tiny particles. 1.1.2 How Small are These Particles? Demonstration: The extreme smallness of particles can be demonstrated through dilution experiments. Example: A few crystals of potassium permanganate ($\text{KMnO}_4$) can color a very large volume of water (e.g., 100 ml $\rightarrow$ 10 ml + 90 ml $\rightarrow$ 1 ml + 9 ml, etc.). Even after 5-8 dilutions, the solution remains colored. Implication: Each crystal of $\text{KMnO}_4$ must contain millions of tiny particles, which keep dividing into smaller and smaller units upon dilution. Scale: Particles of matter are microscopic, often at the atomic or molecular level, far beyond what can be seen with the naked eye. 1.2 Characteristics of Particles of Matter 1.2.1 Particles of Matter Have Space Between Them (Interparticle Space) Evidence: Dissolution: As seen in section 1.1.1, particles of one substance (e.g., sugar) fit into the spaces between particles of another (e.g., water). Diffusion: The intermixing of different types of particles relies on available space. Significance: The amount of interparticle space varies significantly between different states of matter, influencing their properties. 1.2.2 Particles of Matter are Continuously Moving (Kinetic Energy) Intrinsic Motion: Particles of matter are never at rest; they possess kinetic energy and are in constant, random motion. Observation: Example 1: The smell of an unlit incense stick is faint, but a lit one spreads quickly throughout a room. Heat increases particle kinetic energy. Example 2: Dropping a crystal of copper sulphate into water: The blue color slowly spreads even without stirring, due to the movement of copper sulphate and water particles. Example 3: Brownian Motion: The erratic, zigzag movement of microscopic particles suspended in a fluid, caused by collision with the fast-moving fluid molecules. First observed by Robert Brown. Provides strong evidence for the particulate nature and constant motion of matter. Effect of Temperature: Increasing temperature increases the kinetic energy of particles, causing them to move faster. Diffusion: The spontaneous intermixing of particles of different types of matter due to their motion. Occurs faster at higher temperatures. Rate of Diffusion: Gases diffuse fastest, followed by liquids, then solids (which diffuse extremely slowly, if at all, under normal conditions). 1.2.3 Particles of Matter Attract Each Other (Interparticle Forces) Cohesive Forces: There exists a force of attraction between the particles of matter, holding them together. These are also known as intermolecular forces. Strength Variation: The magnitude of this attractive force varies greatly from one type of matter to another and between different states of matter. Example 1: Trying to break an iron nail, a piece of chalk, and a rubber band. The iron nail is hardest to break, implying stronger forces of attraction between its particles. Example 2: A stream of water from a tap can be cut with a finger, but it quickly rejoins. This shows that water particles are held together by attractive forces. Significance: These forces determine the rigidity, fluidity, and other physical properties of substances. 1.3 States of Matter The three common states of matter (Solid, Liquid, Gas) are determined by the interplay of interparticle forces of attraction and the kinetic energy (and thus motion) of the particles. Stronger forces of attraction + Lower kinetic energy $\implies$ Solid Weaker forces of attraction + Higher kinetic energy $\implies$ Gas Intermediate conditions $\implies$ Liquid 1.3.1 The Solid State Characteristics: Definite Shape: Particles are tightly packed in fixed positions. Distinct Boundaries: Maintain their shape even if placed in different containers. Fixed Volume: Occupy a constant amount of space. Rigid: Resist changes to their shape. Incompressible: Particles are already very close, leaving little room for further compression. Low Kinetic Energy: Particles vibrate about their mean positions but do not move freely. Strong Interparticle Forces: Hold particles firmly in place. Slow Diffusion: Very little or no diffusion into other solids (e.g., gold and lead blocks kept in contact for years show minuscule diffusion). Exceptions/Special Cases: Rubber band: Can change shape under external force but regains its original shape when the force is removed. If excessive force is applied, it breaks. This is due to its elastic properties. Sugar/Salt: When kept in different jars, they take the shape of the jar. However, each individual crystal retains its fixed shape. It's a collection of many small solids. Sponge: Is compressible because it has tiny pores in which air is trapped. When pressed, the air is expelled, and it compresses. The solid material of the sponge itself is incompressible. 1.3.2 The Liquid State Characteristics: No Fixed Shape: Takes the shape of the container. Fixed Volume: Occupies a constant amount of space regardless of the container. Fluid: Can flow easily. Not rigid. Slightly Compressible: Particles are closer than in gases but have more space than in solids. Moderate Kinetic Energy: Particles can move and slide past each other. Moderate Interparticle Forces: Strong enough to keep particles close but weak enough to allow movement. Diffusion: Liquids can diffuse into other liquids (e.g., ink in water). Solids and gases can also diffuse into liquids (e.g., sugar in water, oxygen in water). Rate of Diffusion: Higher than solids but lower than gases. Biological Importance: Gases like oxygen ($\text{O}_2$) and carbon dioxide ($\text{CO}_2$) from the atmosphere diffuse and dissolve in water. This dissolved $\text{O}_2$ is vital for the survival of aquatic animals and plants. 1.3.3 The Gaseous State Characteristics: No Fixed Shape: Takes the shape of the container. No Fixed Volume: Occupies the entire volume of the container. Highly Compressible: Particles are far apart with large empty spaces between them. This property is used in LPG (Liquefied Petroleum Gas) and CNG (Compressed Natural Gas). High Kinetic Energy: Particles move randomly and rapidly in all directions, colliding with each other and the container walls. Weak Interparticle Forces: Almost negligible attractive forces between particles. High Diffusion Rate: Gases diffuse very rapidly into other gases (e.g., the smell of food, perfume spreading quickly). Pressure: The random motion and collisions of gas particles with the container walls exert pressure. Examples: The smell of hot sizzling food reaches you several metres away due to the rapid diffusion of gaseous particles with air. LPG used in homes, CNG used as fuel in vehicles are examples of highly compressed gases. 1.4 Can Matter Change its State? Yes, matter can change from one state to another by altering temperature or pressure. Interconversion of States: Solid $\leftrightarrow$ Liquid $\leftrightarrow$ Gas. Water is a classic example: ice (solid), water (liquid), and water vapor (gas). 1.4.1 Effect of Change of Temperature Solid to Liquid (Melting/Fusion): Upon heating a solid, the kinetic energy of its particles increases, causing them to vibrate more vigorously. At a certain temperature, particles gain enough energy to overcome the forces of attraction and break free from their fixed positions, transitioning to a liquid state. Melting Point: The minimum temperature at which a solid melts to become a liquid at atmospheric pressure. Example: Melting point of ice is $0^\circ\text{C}$ or 273.15 K. Latent Heat of Fusion: The amount of heat energy required to change 1 kg of a solid into its liquid state at its melting point without any change in temperature. This "hidden heat" is used to break the interparticle forces. Value for ice: $3.34 \times 10^5 \text{ J/kg}$. Implication: Water at $0^\circ\text{C}$ (273 K) has more energy (due to latent heat) than ice at the same temperature. Liquid to Gas (Boiling/Vaporisation): Upon further heating, liquid particles gain more kinetic energy. At the boiling point, particles have enough energy to overcome all attractive forces and escape into the gaseous state. Boiling Point: The temperature at which a liquid starts boiling at atmospheric pressure. Boiling is a bulk phenomenon, meaning particles from the bulk of the liquid gain enough energy to change to vapor. Example: Boiling point of water is $100^\circ\text{C}$ or 373 K. Latent Heat of Vaporisation: The amount of heat energy required to change 1 kg of a liquid into its gaseous state at its boiling point without any change in temperature. Value for water: $22.5 \times 10^5 \text{ J/kg}$. Implication: Steam at $100^\circ\text{C}$ (373 K) has more energy (due to latent heat) than water at the same temperature, which is why steam causes more severe burns. Gas to Liquid (Condensation/Liquefaction): Cooling a gas removes kinetic energy from particles, allowing attractive forces to pull them closer, forming a liquid. Liquid to Solid (Freezing/Solidification): Cooling a liquid further removes kinetic energy, causing particles to settle into fixed positions, forming a solid. Sublimation: The direct change of state from solid to gas, or from gas to solid (deposition), without passing through the liquid state. Examples: Camphor, naphthalene balls, ammonium chloride, and dry ice (solid $\text{CO}_2$). Experiment: Heating ammonium chloride in a china dish covered with an inverted funnel. Solid $\text{NH}_4\text{Cl}$ sublimes to gas, then deposits as solid on the cooler funnel walls. Temperature Scale Conversion: Celsius to Kelvin: $\text{K} = ^\circ\text{C} + 273.15$ (often rounded to 273). Kelvin to Celsius: $^\circ\text{C} = \text{K} - 273.15$ (often rounded to 273). 0 K is absolute zero, the theoretical lowest possible temperature where particles have minimum kinetic energy. 1.4.2 Effect of Change of Pressure Gases: Applying pressure to a gas reduces the interparticle distance. By applying pressure and reducing temperature, gases can be liquefied. This is how LPG and CNG are produced. Example: Solid Carbon Dioxide ($\text{CO}_2$) or "Dry Ice" is stored under high pressure. If the pressure is reduced to 1 atmosphere, it directly converts to gaseous $\text{CO}_2$ without first melting, hence the name "dry ice." Solids and Liquids: Pressure has a negligible effect on the volume of solids and liquids because their particles are already closely packed. Conclusion: Both temperature and pressure determine the state of matter. 1.5 Evaporation Definition: The phenomenon of a liquid changing into vapor at any temperature below its boiling point. Surface Phenomenon: Unlike boiling (a bulk phenomenon), evaporation occurs only from the surface of the liquid. Mechanism: Particles at the surface of a liquid with higher kinetic energy are able to break free from the attractive forces of other particles and escape into the atmosphere as vapor. 1.5.1 Factors Affecting Evaporation Rate 1. Surface Area: Effect: Increase in surface area increases the rate of evaporation. Reason: More surface particles are exposed to the atmosphere, increasing the chance of escape. Example: Clothes dry faster when spread out. 2. Temperature: Effect: Increase in temperature increases the rate of evaporation. Reason: Higher temperature means more particles have sufficient kinetic energy to overcome attractive forces and escape. Example: Clothes dry faster on a hot day. 3. Humidity: Effect: Decrease in humidity increases the rate of evaporation. Reason: Humidity is the amount of water vapor present in the air. If the air already has a high amount of water vapor, it cannot hold much more, thus decreasing the rate of evaporation. Example: Clothes dry slowly on a humid day. 4. Wind Speed: Effect: Increase in wind speed increases the rate of evaporation. Reason: Increased wind speed moves away the water vapor particles from the liquid's surface, decreasing the concentration of water vapor in the surrounding air. This increases the rate of escape for more water particles. Example: Clothes dry faster on a windy day. 1.5.2 How Does Evaporation Cause Cooling? Principle: During evaporation, the particles that escape from the liquid's surface are the most energetic ones. To compensate for the energy lost by these escaping particles, the remaining liquid particles (and the immediate surroundings) absorb energy from their environment. This absorption of energy from the surroundings makes the surroundings feel cool. Applications/Examples: Acetone/Spirit on Palm: When you put acetone (nail polish remover) or spirit on your palm, it evaporates quickly, taking heat from your palm and making it feel cool. Desert Coolers: Work effectively on hot, dry days. The dry air and high temperature promote rapid evaporation of water from the cooling pads, which absorbs heat from the circulating air, making it cooler. Earthen Pots (Matkas): Keep water cool. The matka has tiny pores through which water seeps to the outer surface and evaporates. This evaporation causes cooling of the remaining water inside. Sweating and Cotton Clothes: In summer, we sweat more. Cotton clothes, being good absorbers of water, absorb sweat and expose it to the atmosphere for easier evaporation. The evaporation of sweat takes latent heat from our body, making us feel cool. Water Droplets on Cold Glass: When ice-cold water is poured into a glass, water vapor present in the air comes in contact with the cold outer surface of the glass. The water vapor loses energy and gets converted into liquid droplets (condensation). Sprinkling Water: People sprinkle water on the roof or open ground after a hot sunny day because the large latent heat of vaporisation of water helps to cool the hot surface. 1.6 Plasma and Bose-Einstein Condensate (BEC) These are two additional states of matter, less common in everyday experience but important in specific conditions. Plasma: Consists of super energetic and super excited particles. These particles are in the form of ionised gases (atoms that have lost or gained electrons). Found in fluorescent tubes and neon sign bulbs. Inside neon sign bulbs, there's neon gas; inside fluorescent tubes, there's helium gas or some other gas. Electricity flowing through them ionises the gas, creating plasma that glows. Also found in stars (including the Sun), where very high temperatures ionise gases to form plasma. Bose-Einstein Condensate (BEC): Predicted by Satyendra Nath Bose and Albert Einstein. Formed by cooling a gas of extremely low density (about one-hundred-thousandth the density of normal air) to super low temperatures, near absolute zero (0 K). At this point, particles lose their individual identities and condense into a single quantum state, behaving as a single "superatom." First achieved by Eric Cornell, Carl Wieman, and Wolfgang Ketterle in 2001. Summary of Key Concepts Matter: Anything with mass and volume, composed of tiny particles. Particle Characteristics: Have spaces between them. Are continuously moving (possess kinetic energy). Attract each other (interparticle forces). Three Common States: Solid: Fixed shape & volume, rigid, incompressible, strong forces, low kinetic energy. Liquid: Fixed volume but no fixed shape, fluid, slightly compressible, moderate forces, moderate kinetic energy. Gas: No fixed shape or volume, highly compressible, fluid, weak forces, high kinetic energy. State Interconversion: Achieved by changing temperature or pressure. Melting/Fusion: Solid $\to$ Liquid (at melting point, absorbs latent heat of fusion). Boiling/Vaporisation: Liquid $\to$ Gas (at boiling point, absorbs latent heat of vaporisation). Condensation: Gas $\to$ Liquid (releases latent heat). Freezing/Solidification: Liquid $\to$ Solid (releases latent heat). Sublimation: Solid $\leftrightarrow$ Gas directly. Evaporation: Liquid $\to$ Gas below boiling point, a surface phenomenon. Factors: Increased surface area, temperature, wind speed; decreased humidity. Cooling Effect: Evaporation takes latent heat from surroundings, causing cooling. Other States: Plasma (ionised gas) and Bose-Einstein Condensate (super-cooled, super-low density gas). Measurable Quantities and SI Units Quantity SI Unit Symbol Conversion Example Temperature kelvin K $^\circ\text{C} + 273.15 \approx \text{K}$ Length metre m 1 km = 1000 m Mass kilogram kg 1 g = 0.001 kg Weight newton N Volume cubic metre $\text{m}^3$ 1 L = $1 \text{ dm}^3 = 10^{-3} \text{ m}^3$ Density kilogram per cubic metre $\text{kg m}^{-3}$ Pressure pascal Pa 1 atm $\approx 1.01 \times 10^5 \text{ Pa}$ Energy/Heat joule J 1 calorie = 4.184 J