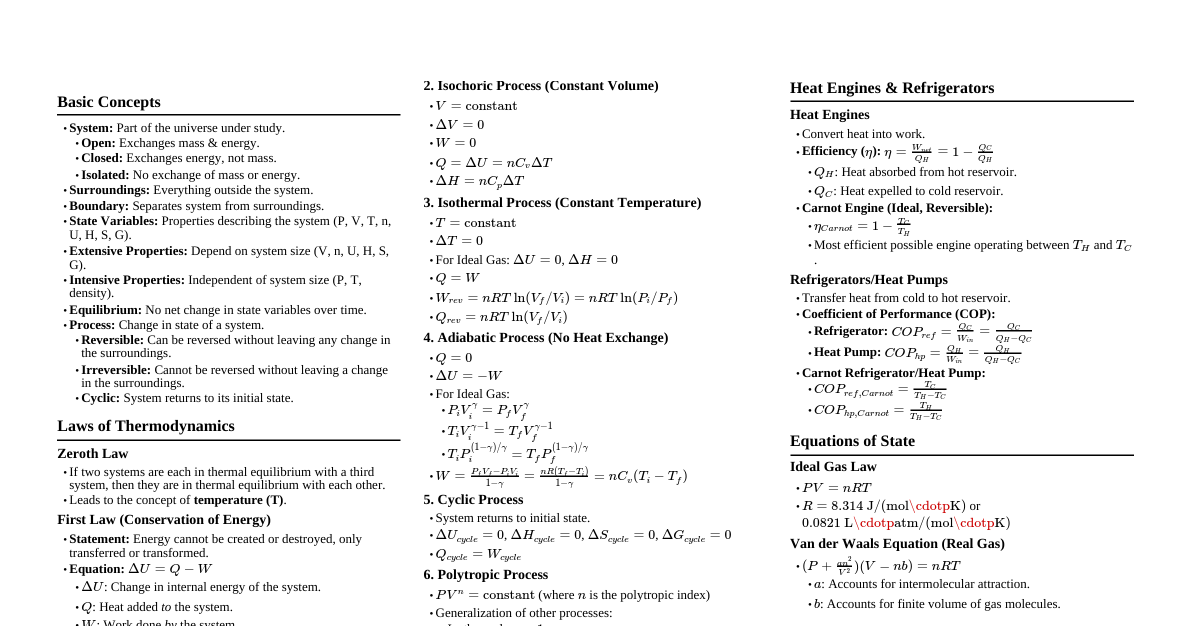
### Introduction to Thermodynamics - **Thermodynamics:** Study of heat and its relation to other forms of energy and work. - **System:** Part of the universe under consideration. - **Surroundings:** Everything outside the system. - **Boundary:** Separates system from surroundings. - **Types of Systems:** - **Open:** Exchanges both mass and energy. - **Closed:** Exchanges energy but not mass. - **Isolated:** Exchanges neither mass nor energy. - **State Functions:** Properties that depend only on the initial and final states of the system, not the path taken (e.g., U, H, S, G, P, V, T). - **Path Functions:** Properties that depend on the path taken (e.g., q, w). ### Zeroth Law of Thermodynamics - **Statement:** If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. - **Significance:** Defines temperature as a fundamental property and allows for its measurement. - **Concept:** All systems in thermal equilibrium have the same temperature. ### First Law of Thermodynamics (Conservation of Energy) - **Statement:** Energy cannot be created or destroyed, only transferred or transformed. - **Mathematical Form:** $\Delta U = q + w$ - $\Delta U$: Change in internal energy of the system. - $q$: Heat added to the system (positive if absorbed, negative if released). - $w$: Work done on the system (positive if done on system, negative if done by system). - **Internal Energy (U):** Sum of all kinetic and potential energies of the particles within a system. - **Work (w):** - **Pressure-Volume Work:** $w = -P_{ext}\Delta V$ (for constant external pressure). - If reversible, $w = -\int P dV$. - **Enthalpy (H):** - **Definition:** $H = U + PV$ - **Change in Enthalpy:** $\Delta H = \Delta U + \Delta(PV)$ - For reactions at constant pressure: $\Delta H = q_p$ (heat exchanged at constant pressure). - **Exothermic:** $\Delta H 0$ (absorbs heat). - **Heat Capacity (C):** - $q = C\Delta T$ - $C_v = (\frac{\partial U}{\partial T})_V$ (constant volume) - $C_p = (\frac{\partial H}{\partial T})_P$ (constant pressure) - For ideal gases: $C_p - C_v = R$ ### Second Law of Thermodynamics (Entropy and Spontaneity) - **Statement:** The total entropy of an isolated system can only increase over time, or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. - **Mathematical Form:** $\Delta S_{universe} = \Delta S_{system} + \Delta S_{surroundings} \ge 0$ - For a reversible process: $\Delta S = \frac{q_{rev}}{T}$ - For an irreversible (spontaneous) process: $\Delta S > \frac{q_{irr}}{T}$ - **Entropy (S):** Measure of the disorder or randomness of a system. - **Key Principles:** - Heat flows spontaneously from hotter to colder bodies. - It's impossible to convert heat completely into work in a cyclic process (Kelvin-Planck statement). - It's impossible to construct a device that solely transfers heat from a colder to a hotter body without external work (Clausius statement). - **Standard Molar Entropy ($S^\circ$):** Entropy of one mole of a substance at standard conditions (298 K, 1 atm). - **Change in Entropy for a Reaction:** $\Delta S^\circ_{rxn} = \sum n S^\circ_{products} - \sum m S^\circ_{reactants}$ ### Third Law of Thermodynamics - **Statement:** The entropy of a perfect crystal at absolute zero (0 Kelvin) is exactly zero. - **Significance:** Provides a reference point for calculating absolute entropies. - **Implication:** It is impossible to reach absolute zero in a finite number of steps. ### Gibbs Free Energy (G) and Spontaneity - **Definition:** A thermodynamic potential that measures the "useful" or process-initiating work obtainable from an isothermal, isobaric thermodynamic system. - **Mathematical Form:** $G = H - TS$ - **Change in Gibbs Free Energy:** $\Delta G = \Delta H - T\Delta S$ (at constant temperature) - **Criteria for Spontaneity (at constant T and P):** - $\Delta G 0$: Process is non-spontaneous (endergonic); the reverse process is spontaneous. - **Standard Gibbs Free Energy Change ($\Delta G^\circ$):** - **From Standard Enthalpies and Entropies:** $\Delta G^\circ = \Delta H^\circ - T\Delta S^\circ$ - **From Standard Free Energies of Formation:** $\Delta G^\circ_{rxn} = \sum n \Delta G^\circ_f(products) - \sum m \Delta G^\circ_f(reactants)$ - **Gibbs Free Energy and Equilibrium Constant (K):** - $\Delta G = \Delta G^\circ + RT \ln Q$ (where Q is reaction quotient) - At equilibrium, $\Delta G = 0$ and $Q = K$: $\Delta G^\circ = -RT \ln K$ - **Relationship between $\Delta G^\circ$ and K:** - If $\Delta G^\circ 1$ (products favored at equilibrium). - If $\Delta G^\circ = 0$, $K = 1$ (reactants and products equally favored). - If $\Delta G^\circ > 0$, $K ### Maxwell Relations - **Purpose:** Relate partial derivatives of thermodynamic properties. - **Fundamental Thermodynamic Relations:** - $dU = TdS - PdV$ - $dH = TdS + VdP$ - $dG = VdP - SdT$ - $dA = -PdV - SdT$ (Helmholtz Free Energy $A = U - TS$) - **Maxwell Relations (from exact differentials):** - $(\frac{\partial T}{\partial V})_S = -(\frac{\partial P}{\partial S})_V$ - $(\frac{\partial T}{\partial P})_S = (\frac{\partial V}{\partial S})_P$ - $(\frac{\partial V}{\partial T})_P = -(\frac{\partial S}{\partial P})_T$ - $(\frac{\partial P}{\partial T})_V = (\frac{\partial S}{\partial V})_T$ ### Phase Transitions and Equilibrium - **Clausius-Clapeyron Equation:** Describes the relationship between pressure, temperature, and enthalpy of vaporization/sublimation for a phase transition. - $\frac{dP}{dT} = \frac{\Delta H}{T\Delta V}$ - For liquid-vapor or solid-vapor (assuming ideal gas and $\Delta V \approx V_{gas}$): $\ln(\frac{P_2}{P_1}) = -\frac{\Delta H_{vap/sub}}{R}(\frac{1}{T_2} - \frac{1}{T_1})$ - **Phase Diagram:** Graph showing the stable phases of a substance at different temperatures and pressures. - **Triple Point:** Temperature and pressure where all three phases (solid, liquid, gas) coexist in equilibrium. - **Critical Point:** Temperature and pressure above which distinct liquid and gas phases do not exist (supercritical fluid). ### Introduction to Statistical Thermodynamics - **Bridge between Micro and Macro:** Connects microscopic properties of atoms/molecules to macroscopic thermodynamic properties. - **Boltzmann Equation for Entropy:** $S = k_B \ln W$ - $k_B$: Boltzmann constant ($1.38 \times 10^{-23} J/K$) - $W$: Number of microstates corresponding to a given macrostate (multiplicity). - **Partition Function (Q):** - $Q = \sum_i e^{-E_i / k_B T}$ (for a single molecule) - $Q_{total} = q_{trans} q_{rot} q_{vib} q_{elec}$ (for total molecular partition function) - **Connecting Q to Thermodynamic Properties:** - $U = k_B T^2 (\frac{\partial \ln Q}{\partial T})_V$ - $S = k_B \ln Q + k_B T (\frac{\partial \ln Q}{\partial T})_V$ - $P = k_B T (\frac{\partial \ln Q}{\partial V})_T$ - $A = -k_B T \ln Q$