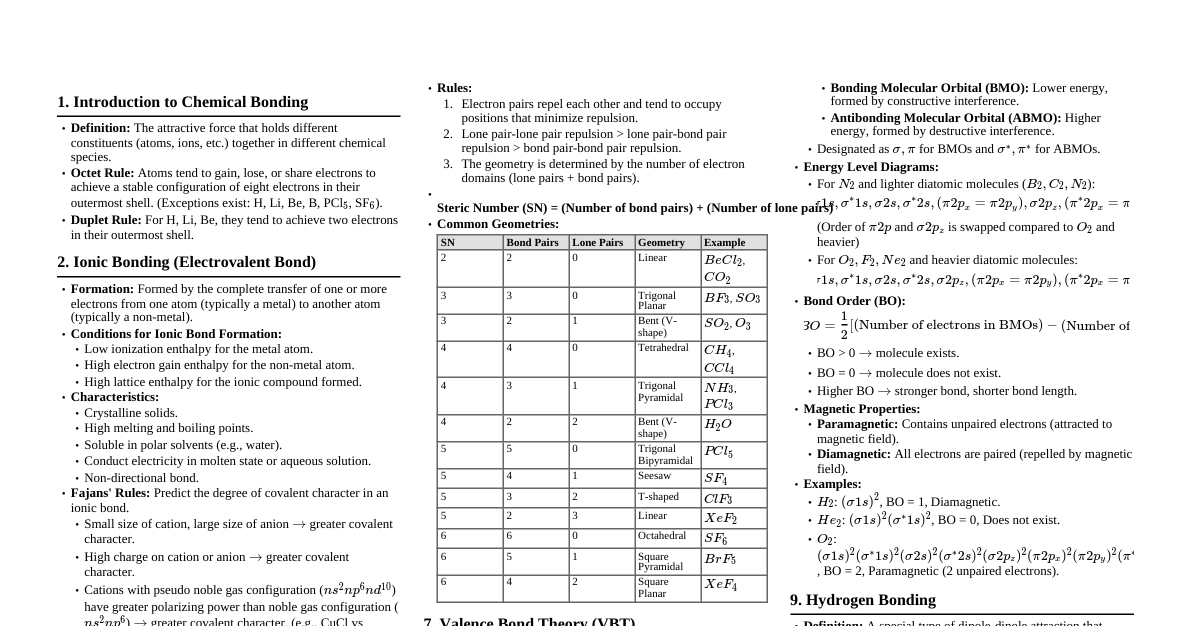
1. Types of Chemical Bonds A. Ionic Bond (Electrovalent) Definition: Complete transfer of electrons, forming ions held by electrostatic force. Formation: Metal loses $e^-$ (cation), Non-metal gains $e^-$ (anion). Example: $\text{Ca}^{2+} + 2\text{Cl}^- \rightarrow \text{CaCl}_2$ Characteristics: Between metals & non-metals Non-directional High MP/BP Conducts in molten/aqueous state Soluble in water B. Covalent Bond Definition: Mutual sharing of electrons. Formation: Atoms share electrons to achieve noble gas configuration. Example: $\text{Cl}_2$ molecule (Cl-Cl) Types (by shared pairs): Type Electrons Shared Example Single Bond 2 (1 pair) H-H, Cl-Cl Double Bond 4 (2 pairs) O=O, C=O Triple Bond 6 (3 pairs) N≡N, C≡C Characteristics: Between non-metals Directional Lower MP/BP Poor conductors Soluble in non-polar solvents C. Coordinate (Dative) Bond Definition: Special covalent bond where both electrons come from one atom (donor). Requirements: Donor (lone pair) & Acceptor (vacant orbital). Example: $\text{NH}_3 \rightarrow \text{BF}_3$ (N donates lone pair to B) Key Point: Identical to regular covalent bond once formed. 2. Lewis Structures Rules: Count total valence electrons. Draw skeletal structure (least electronegative atom central). Complete octets of outer atoms. Place remaining electrons on central atom. Form multiple bonds if central atom lacks octet. Examples: $\text{H}_2\text{O}$: H-O-H (with 2 lone pairs on O) $\text{CO}_2$: O=C=O $\text{CH}_4$: Tetrahedral structure 3. Octet Rule Statement: Atoms tend to achieve 8 valence electrons. Limitations: A. Incomplete Octet Atoms with < 8 electrons but stable: Compound Central Atom Valence Electrons LiCl Li 2 BeCl$_2$ Be 4 BF$_3$ B 6 B. Expanded Octet Atoms with > 8 electrons: Compound Central Atom Valence Electrons PCl$_5$ P 10 SF$_6$ S 12 H$_2$SO$_4$ S 12 4. Bond Parameters A. Bond Length Definition: Equilibrium distance between nuclei. Factors: Bond order (higher $\rightarrow$ shorter), Atom size (larger $\rightarrow$ longer). Order: Single > Double > Triple Example: C-C (1.54 Å) > C=C (1.34 Å) > C≡C (1.20 Å) B. Bond Enthalpy (Bond Energy) Definition: Energy to break one mole of bond in gaseous state. Characteristics: Measured in kJ/mol. Higher enthalpy $\rightarrow$ Stronger bond. Order: Triple > Double > Single C. Bond Order Formula: $\text{Bond Order} = (\text{N}_b - \text{N}_a)/2$ $\text{N}_b$ = bonding electrons, $\text{N}_a$ = antibonding electrons. Significance: Higher bond order $\rightarrow$ Stronger and shorter bond, more stable molecule. 5. Types of Orbital Overlap Formation of $\sigma$ (Sigma) Bonds 1. s-s Overlap: Between two s orbitals (e.g., $\text{H}_2$). 2. s-p Overlap: Between s and p orbital (e.g., HF). 3. p-p Overlap: Between two p orbitals (axial) (e.g., $\text{F}_2$). Formation of $\pi$ (Pi) Bonds Lateral p-p Overlap: Sideways overlap of p orbitals, perpendicular to internuclear axis (e.g., C=C). 6. $\sigma$ vs $\pi$ Bonds Feature $\sigma$ (Sigma) Bond $\pi$ (Pi) Bond Overlap Type Axial (head-on) Lateral (sideways) Electron Density High along axis Zero along axis Strength Strong Weak Rotation Free rotation Restricted Formation First bond Second/third bond 7. Hybridization Definition: Mixing of atomic orbitals to form new equivalent hybrid orbitals. Need: Explains valency and geometry (e.g., $\text{CH}_4$ tetrahedral). Repulsion Order: Lone pair-Lone pair > Lone pair-Bond pair > Bond pair-Bond pair Effect of Lone Pairs: More lone pairs $\rightarrow$ More compression $\rightarrow$ Smaller angle. Types of Hybridization A. sp Hybridization: 1 s + 1 p orbital $\rightarrow$ 2 sp hybrid orbitals. Linear geometry, 180° bond angle. Example: $\text{BeCl}_2$, $\text{C}_2\text{H}_2$. B. $\text{sp}^2$ Hybridization: 1 s + 2 p orbitals $\rightarrow$ 3 $\text{sp}^2$ hybrid orbitals. Trigonal planar geometry, 120° bond angle. Example: $\text{BF}_3$, $\text{C}_2\text{H}_4$. C. $\text{sp}^3$ Hybridization: 1 s + 3 p orbitals $\rightarrow$ 4 $\text{sp}^3$ hybrid orbitals. Tetrahedral geometry, 109°28' bond angle. Example: $\text{CH}_4$, $\text{NH}_3$, $\text{H}_2\text{O}$. Effect of Lone Pairs on Bond Angles: Molecule Hybridization Lone Pairs Bond Pairs Expected Angle Actual Angle CH$_4$ sp$^3$ 0 4 109°28' 109°28' NH$_3$ sp$^3$ 1 3 109°28' 107°18' H$_2$O sp$^3$ 2 2 109°28' 104°35' 8. VSEPR Theory Full Form: Valence Shell Electron Pair Repulsion Theory. Postulates: Electron pairs repel each other. They arrange to be maximum apart. Lone pairs repel more strongly than bond pairs. Multiple bonds treated as single electron pair. Predicting Geometry: $\text{AX}_n\text{E}_m$ (A=central, X=bonded, E=lone pairs). Common Geometries: Formula Bond Pairs Lone Pairs Geometry Example Bond Angle AX$_2$ 2 0 Linear CO$_2$, BeCl$_2$ 180° AX$_3$ 3 0 Trigonal planar BF$_3$ 120° AX$_2$E 2 1 Bent SO$_2$ <120° AX$_4$ 4 0 Tetrahedral CH$_4$ 109°28' AX$_3$E 3 1 Pyramidal NH$_3$ 107°18' AX$_2$E$_2$ 2 2 Bent/V-shaped H$_2$O 104°35' AX$_5$ 5 0 Trigonal bipyramidal PCl$_5$ 90°, 120° AX$_6$ 6 0 Octahedral SF$_6$ 90° 9. Polarity of Molecules Polar Covalent Bond Between atoms with different electronegativities. Unequal sharing, partial charges ($\delta^+$, $\delta^-$). Has dipole moment ($\mu$). Example: H-F. Non-polar Covalent Bond Between identical atoms or atoms with same electronegativity. Equal sharing, no charge separation. Zero dipole moment. Examples: $\text{H}_2$, $\text{Cl}_2$, $\text{N}_2$. Polar vs Non-polar Molecules: Depends on bond polarity and molecular geometry. Molecule Bond Type Geometry Net Dipole Polarity CO$_2$ Polar Linear Zero (cancel out) Non-polar H$_2$O Polar Bent Non-zero Polar CH$_4$ Polar Tetrahedral Zero (symmetric) Non-polar NH$_3$ Polar Pyramidal Non-zero Polar 10. Molecular Orbital Theory (MOT) Key Concepts: Molecular orbitals (MOs) formed by combination of atomic orbitals. MOs belong to entire molecule. Types of MOs: A. Bonding Molecular Orbitals (BMO): Constructive interference, lower energy, increased electron density. ($\sigma, \pi$) B. Antibonding Molecular Orbitals (ABMO): Destructive interference, higher energy, decreased electron density. ($\sigma^*, \pi^*$) Electronic Configuration of $\text{O}_2$: $(\sigma1s)^2 (\sigma^*1s)^2 (\sigma2s)^2 (\sigma^*2s)^2 (\sigma2p_z)^2 (\pi2p_x)^2 (\pi2p_y)^2 (\pi^*2p_x)^1 (\pi^*2p_y)^1$ Bond Order of $\text{O}_2$: $(10-6)/2 = 2$. Paramagnetism of $\text{O}_2$: Contains 2 unpaired electrons, hence paramagnetic. 11. Formal Charge Formula: $\text{FC} = \text{V} - \text{N} - \text{B}/2$ V = Valence electrons in free atom N = Non-bonding electrons (lone pairs) B = Bonding electrons (shared) Rules for Best Structure: Lowest formal charges preferred. Formal charges closest to zero. Negative formal charge on more electronegative atom. Adjacent atoms should not have same sign charges. Memory Tricks & Techniques 1. Hybridization Quick Check: Geometry Hybridization Angle Linear sp 180° Trigonal sp$^2$ 120° Tetrahedral sp$^3$ 109°28' 2. Bond Strength Ladder: Triple > Double > Single > Hydrogen bond > Van der Waals. 3. Octet Exceptions - "BIG FIVE": Boron (6e$^-$), Iodine (14e$^-$ in IF$_7$), Group 15 expanded (P, S - 10, 12e$^-$). 4. Lone Pair Effect - "LAB Rule": L one pairs A re B ullies (push bond pairs, reduce angles). 5. Sigma-Pi Quick ID: First bond = $\sigma$. Additional bonds = $\pi$. Single = $1\sigma$. Double = $1\sigma + 1\pi$. Triple = $1\sigma + 2\pi$. 6. Electronegativity Trend: F > O > N > Cl > Br > I > C > H. 7. Bond Length vs Strength: Shorter bond = Stronger bond = Higher energy. Important Comparisons Table Property Ionic Covalent Coordinate Electron transfer Complete Sharing Sharing (from one) Nature Non-directional Directional Directional Melting point High Low Moderate Conductivity Good (molten/aq) Poor Poor Rapid Revision Points Octet rule = 8 electrons (exceptions: H=2, Be=4, B=6, P/S>8). Hybridization = Mixing orbitals $\rightarrow$ explains geometry. VSEPR = Electron pairs repel $\rightarrow$ maximum separation. Bond order $\propto$ Strength $\propto$ 1/Length. $\sigma$ bond = Strong, axial | $\pi$ bond = Weak, lateral. Polar molecule = Asymmetric + different atoms. $\text{O}_2$ paramagnetic = 2 unpaired electrons (MOT explains). Formal charge = Helps find best Lewis structure. Exam-Focused Formulas $\text{Bond Order} = (\text{N}_b - \text{N}_a)/2$ $\text{Formal Charge} = \text{V} - \text{N} - \text{B}/2$ $\text{Dipole Moment} (\mu) = \text{Q} \times \text{d}$ $\text{% Ionic character} = (\text{Observed dipole}/\text{Theoretical dipole}) \times 100$