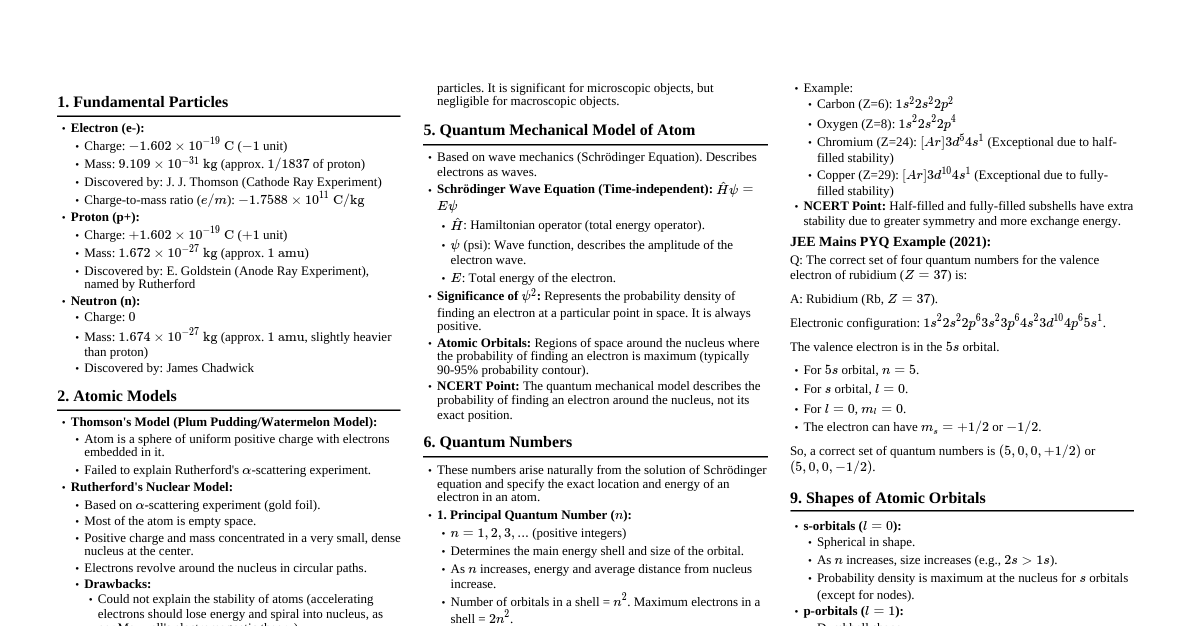
1. Introduction to Coordination Compounds Definition: Compounds formed from a central metal atom/ion bonded to a group of ions or molecules (ligands) by coordinate bonds. Double Salts vs. Coordination Compounds: Double Salts: Retain their identity in solid state but dissociate completely into constituent ions in solution (e.g., Carnallite, Mohr's salt). Coordination Compounds: Retain their identity in both solid state and solution (e.g., $K_4[Fe(CN)_6]$). 2. Terminology Central Metal Atom/Ion: A Lewis acid that accepts electron pairs from ligands. Usually d-block elements. Ligands: Lewis bases that donate electron pairs to the central metal atom/ion. Classification by Denticity: Monodentate: Donate one electron pair (e.g., $H_2O$, $NH_3$, $Cl^-$, $CN^-$). Bidentate: Donate two electron pairs (e.g., ethylenediamine (en), oxalate ($C_2O_4^{2-}$)). Polydentate: Donate multiple electron pairs (e.g., EDTA (hexadentate)). Ambidentate Ligands: Can coordinate through two different atoms (e.g., $NO_2^-$ (N or O), $SCN^-$ (S or N)). Coordination Number (CN): The number of ligand donor atoms directly bonded to the central metal ion. Coordination Sphere: The central metal ion and the ligands directly attached to it, enclosed in square brackets $[ \dots ]$. Counter Ions: Ions outside the coordination sphere that balance the charge of the complex ion. Chelate Ligands: Bidentate or polydentate ligands that form ring structures with the metal ion (chelation). Chelate complexes are more stable (chelate effect). 3. Werner's Theory of Coordination Compounds Primary Valency: Ionizable valency, satisfied by anions, corresponds to the oxidation state of the metal. Secondary Valency: Non-ionizable valency, satisfied by ligands, corresponds to the coordination number. Fixed for a metal. Secondary valencies are directed in space, giving a definite geometry to the complex. 4. Nomenclature (IUPAC) Cation named first, then anion. Ligands named before the metal. Ligands name: Anionic ligands end in '-o' (e.g., $Cl^-$: chloro, $OH^-$: hydroxo, $CN^-$: cyano). Neutral ligands often have special names ($H_2O$: aqua, $NH_3$: ammine, $CO$: carbonyl, $NO$: nitrosyl). Prefixes di-, tri-, tetra- for simple ligands. For complex ligands (e.g., 'en', 'triphosphine'), use bis-, tris-, tetrakis-. Oxidation state of metal in Roman numerals in parentheses. If complex is an anion, metal name ends in '-ate' (e.g., Ferrate, Cuprate, Argentate). Alphabetical order of ligands. Examples: $[Co(NH_3)_6]Cl_3$: Hexaamminecobalt(III) chloride $K_4[Fe(CN)_6]$: Potassium hexacyanoferrate(II) $[Pt(NH_3)_2Cl_2]$: Diamminedichloroplatinum(II) 5. Isomerism in Coordination Compounds A. Structural Isomerism Ionization Isomerism: Differ in the ionizable counter ion and a ligand (e.g., $[Co(NH_3)_5Br]SO_4$ and $[Co(NH_3)_5SO_4]Br$). Hydrate Isomerism: Water molecule acts as a ligand or as a molecule of crystallization (e.g., $[Cr(H_2O)_6]Cl_3$, $[Cr(H_2O)_5Cl]Cl_2 \cdot H_2O$). Linkage Isomerism: Ambidentate ligand coordinates through different donor atoms (e.g., $[Co(NH_3)_5NO_2]^{2+}$ (nitro) and $[Co(NH_3)_5ONO]^{2+}$ (nitrito)). Coordination Isomerism: Exchange of ligands between cationic and anionic coordination spheres (e.g., $[Co(NH_3)_6][Cr(CN)_6]$ and $[Cr(NH_3)_6][Co(CN)_6]$). B. Stereoisomerism Geometrical Isomerism (cis-trans): Different arrangements of ligands around the central metal ion. $MA_2B_2$ (Square Planar): cis and trans. $MA_4B_2$ (Octahedral): cis and trans. $MA_3B_3$ (Octahedral): facial (fac) and meridional (mer). Optical Isomerism (Enantiomerism): Non-superimposable mirror images. Occurs in chiral complexes. Common in octahedral complexes, especially those with bidentate ligands (e.g., $[Co(en)_3]^{3+}$). Square planar complexes usually don't show optical isomerism. 6. Valence Bond Theory (VBT) Metal-ligand bond is coordinate, formed by overlap of filled ligand orbital and vacant metal hybrid orbital. Predicts geometry based on hybridization. Hybridization and Geometry: $sp^3$: Tetrahedral $dsp^2$: Square planar $sp^3d^2$: Octahedral (outer orbital complex, high spin) $d^2sp^3$: Octahedral (inner orbital complex, low spin) Magnetic Properties: Paramagnetic: Presence of unpaired electrons. Diamagnetic: No unpaired electrons. Strong Field Ligands: Cause large crystal field splitting, force pairing of electrons, form low spin complexes (e.g., $CN^-$, $CO$, $en$, $NO_2^-$). Weak Field Ligands: Cause small crystal field splitting, electrons remain unpaired, form high spin complexes (e.g., $H_2O$, $OH^-$, $Cl^-$, $F^-$, $Br^-$, $I^-$). Limitations: Doesn't explain color. Doesn't distinguish between strong and weak field ligands theoretically. Doesn't give quantitative results for magnetic properties. 7. Crystal Field Theory (CFT) Treats metal-ligand bond as purely ionic (electrostatic interaction). Ligands are point charges. Crystal Field Splitting: Degeneracy of d-orbitals is lifted in the presence of ligands. Octahedral Field: d-orbitals split into two sets: $t_{2g}$ (lower energy, $d_{xy}, d_{yz}, d_{zx}$) $e_g$ (higher energy, $d_{x^2-y^2}, d_{z^2}$) Energy difference is $\Delta_o$ or $10Dq$. Tetrahedral Field: d-orbitals split into two sets: $e$ (lower energy, $d_{x^2-y^2}, d_{z^2}$) $t_2$ (higher energy, $d_{xy}, d_{yz}, d_{zx}$) Energy difference is $\Delta_t$. Note: $\Delta_t \approx \frac{4}{9}\Delta_o$. Always high spin complexes. Crystal Field Stabilization Energy (CFSE): Energy stabilization due to splitting of d-orbitals. Spectrochemical Series: An ordering of ligands based on their ability to cause crystal field splitting: $I^- (Weak field $\to$ Strong field) Color of Coordination Compounds: Due to d-d transitions (absorption of light in visible region, complementary color observed). Limitations: Purely ionic model is unrealistic. Doesn't explain bonding in carbonyls well. Doesn't account for $\pi$-bonding in ligands. 8. Metal Carbonyls Compounds formed between transition metals and carbon monoxide (CO) ligands. CO is a strong field ligand. Synergic Bonding: Involves both $\sigma$ donation from CO to metal and $\pi$ back-bonding from metal d-orbitals to CO $\pi^*$ antibonding orbitals. This strengthens both M-C and C-O bonds. Usually have low oxidation states for the metal. Examples: $Ni(CO)_4$ (tetrahedral), $Fe(CO)_5$ (trigonal bipyramidal), $Cr(CO)_6$ (octahedral).