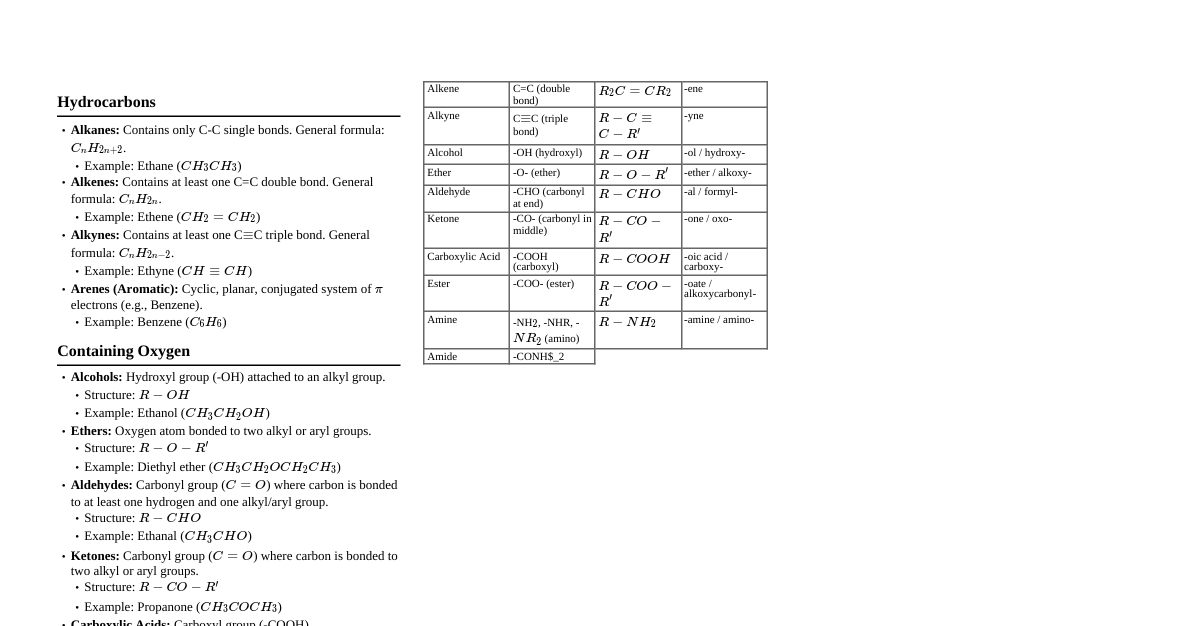
### Basics of Organic Chemistry - **Definition:** Study of carbon compounds. Carbon forms four bonds (tetravalency) due to its electronic configuration ($1s^2 2s^2 2p^2$). - **Catenation:** Carbon's unique ability to form long chains and rings with other carbon atoms. - **Hybridization:** - $sp^3$: Single bonds (e.g., alkanes), tetrahedral geometry, bond angle $\approx 109.5^\circ$. - $sp^2$: Double bonds (e.g., alkenes), trigonal planar geometry, bond angle $\approx 120^\circ$. - $sp$: Triple bonds (e.g., alkynes), linear geometry, bond angle $\approx 180^\circ$. - **Functional Groups:** Specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. Examples: - Alcohols (-OH) - Aldehydes (-CHO) - Ketones (C=O within chain) - Carboxylic Acids (-COOH) ### Isomerism - **Definition:** Compounds having the same molecular formula but different structural formulas or spatial arrangements. - **Structural Isomerism:** Different connectivity of atoms. - **Chain Isomerism:** Different carbon skeleton (e.g., n-butane vs. isobutane). - **Position Isomerism:** Different position of functional group or substituent (e.g., 1-propanol vs. 2-propanol). - **Functional Group Isomerism:** Different functional groups (e.g., ethanol vs. dimethyl ether). - **Metamerism:** Different alkyl groups attached to the same functional group (e.g., diethyl ether vs. methyl propyl ether). - **Tautomerism:** Rapid equilibrium between two functional isomers (e.g., keto-enol tautomerism). - **Stereoisomerism:** Same connectivity but different spatial arrangement. - **Geometrical (cis-trans) Isomerism:** Restricted rotation around a double bond or in a cyclic structure. - **cis:** Same groups on the same side. - **trans:** Same groups on opposite sides. - **Optical Isomerism:** Non-superimposable mirror images (enantiomers) due to chiral centers. - **Chiral Carbon:** Carbon atom bonded to four different groups. - **Enantiomers:** Optical isomers that are mirror images. - **Diastereomers:** Stereoisomers that are not mirror images. - **Racemic Mixture:** An equimolar mixture of enantiomers; optically inactive. ### Purification and Analysis - **Purification Techniques:** - **Crystallization:** For solids, based on difference in solubility. - **Distillation:** For liquids, based on difference in boiling points. - Simple distillation, Fractional distillation, Vacuum distillation, Steam distillation. - **Chromatography:** Separation based on differential adsorption/partition between stationary and mobile phases. - Paper, Thin Layer (TLC), Column, Gas Chromatography (GC), High-Performance Liquid Chromatography (HPLC). - **Differential Extraction:** Separation using immiscible solvents. - **Qualitative Analysis:** - **Lassaigne's Test:** Detection of N, S, P, halogens. - Detection of C and H (by heating with CuO). - **Quantitative Analysis:** - **Dumas Method:** For Nitrogen. - **Kjeldahl Method:** For Nitrogen (not for nitro, azo, or ring N). - **Carius Method:** For Halogens and Sulfur. - **Liebig's Method:** For Carbon and Hydrogen. - Estimation of Oxygen (by difference). ### Alkanes - **General Formula:** $C_nH_{2n+2}$ (Saturated hydrocarbons). - **Nomenclature:** IUPAC rules (longest chain, lowest numbers for substituents). - **Preparation:** - Hydrogenation of alkenes/alkynes (Sabatier-Senderens reaction). - Wurtz reaction: $2RX + 2Na \xrightarrow{\text{dry ether}} R-R + 2NaX$ (for even number carbons). - Decarboxylation of carboxylic acids (Soda-lime method). - Kolbe's electrolytic method. - Reduction of alkyl halides. - **Reactions:** - **Substitution Reactions:** Free radical halogenation (e.g., chlorination, bromination). - Mechanism: Initiation, Propagation, Termination. - Combustion. - Controlled Oxidation. - Isomerization. - Aromatization. - Pyrolysis (cracking). ### Alkenes - **General Formula:** $C_nH_{2n}$ (Unsaturated hydrocarbons, contain C=C double bond). - **Nomenclature:** IUPAC rules (double bond gets lowest possible number). - **Preparation:** - Dehydration of alcohols (acid-catalyzed). - Dehydrohalogenation of alkyl halides (Saytzeff's rule). - Dehalogenation of vicinal dihalides. - Partial hydrogenation of alkynes (Lindlar's catalyst for cis-alkene). - Kolbe's electrolytic method. - **Reactions:** - **Electrophilic Addition Reactions:** Characteristic reaction. - Addition of $H_2$ (hydrogenation). - Addition of halogens ($X_2$). - Addition of $HX$ (hydrohalogenation) - **Markovnikov's Rule:** H adds to the carbon with more H atoms. - Addition of $H_2O$ (hydration) - acid catalyzed. - Addition of $HBr$ in presence of peroxide - **Anti-Markovnikov's Rule** (Kharasch effect). - Oxidation: Baeyer's reagent (cold, dilute, alkaline $KMnO_4$), Ozonolysis. - Polymerization. ### Alkynes - **General Formula:** $C_nH_{2n-2}$ (Unsaturated hydrocarbons, contain C≡C triple bond). - **Nomenclature:** IUPAC rules (triple bond gets lowest possible number). - **Preparation:** - Dehydrohalogenation of vicinal or geminal dihalides. - From calcium carbide ($CaC_2 + 2H_2O \rightarrow Ca(OH)_2 + C_2H_2$). - Kolbe's electrolytic method. - **Reactions:** - **Electrophilic Addition Reactions:** Similar to alkenes, but two molecules can add across the triple bond. - Addition of $H_2$, $X_2$, $HX$, $H_2O$ (hydration, forms ketones/aldehydes via enol tautomerism). - Acidity of terminal alkynes: The H atom on $sp$-hybridized carbon is acidic. Reacts with Na, NaNH$_2$, ammoniacal $AgNO_3$ (Tollens' reagent), ammoniacal $CuCl$ (Fehling's solution). - Polymerization: Linear (e.g., acetylene to polyacetylene) and cyclic (e.g., acetylene to benzene). ### Aromatic Hydrocarbons (Benzene) - **Aromaticity (Hückel's Rule):** Planar, cyclic, fully conjugated system with $(4n+2)\pi$ electrons ($n=0, 1, 2, ...$). - **Benzene ($C_6H_6$):** Aromatic, resonance stabilized. - **Preparation:** - Cyclic polymerization of ethyne (acetylene). - Decarboxylation of aromatic acids. - Reduction of phenol. - **Reactions:** - **Electrophilic Substitution Reactions:** Characteristic reaction. - Nitration: With $HNO_3/H_2SO_4$. - Halogenation: With $X_2/FeX_3$. - Sulfonation: With fuming $H_2SO_4$. - Friedel-Crafts Alkylation: With $RCl/AlCl_3$. - Friedel-Crafts Acylation: With $RCOCl/AlCl_3$. - **Directive Influence of Substituents:** - **Ortho-para directing & Activating:** -OH, -NH$_2$, -OR, -R, -X (halogens are deactivating but o,p-directing). - **Meta directing & Deactivating:** -NO$_2$, -COOH, -CHO, -CN, -SO$_3H$. - Addition Reactions (under harsh conditions): Hydrogenation, Halogenation (e.g., BHC). - Combustion. ### Reaction Mechanisms - **Homolytic Fission:** Bond breaks symmetrically, forming free radicals. - **Heterolytic Fission:** Bond breaks unsymmetrically, forming carbocations or carbanions. - **Electrophiles:** Electron-deficient species, accept electron pair (Lewis acids). - **Nucleophiles:** Electron-rich species, donate electron pair (Lewis bases). - **Types of Organic Reactions:** - **Substitution:** One atom/group replaced by another. - **Addition:** Atoms/groups added across multiple bonds. - **Elimination:** Atoms/groups removed to form multiple bonds. - **Rearrangement:** Atoms/groups migrate within the molecule. - **Electronic Effects:** - **Inductive Effect (+I/-I):** Permanent displacement of $\sigma$-electrons along a chain due to electronegativity difference. - **Resonance Effect (+R/-R or Mesomeric Effect):** Delocalization of $\pi$-electrons in conjugated systems. - **Hyperconjugation (No-bond Resonance):** Delocalization of $\sigma$-electrons of C-H bond with adjacent $\pi$-system or empty p-orbital (stabilizes carbocations and free radicals).