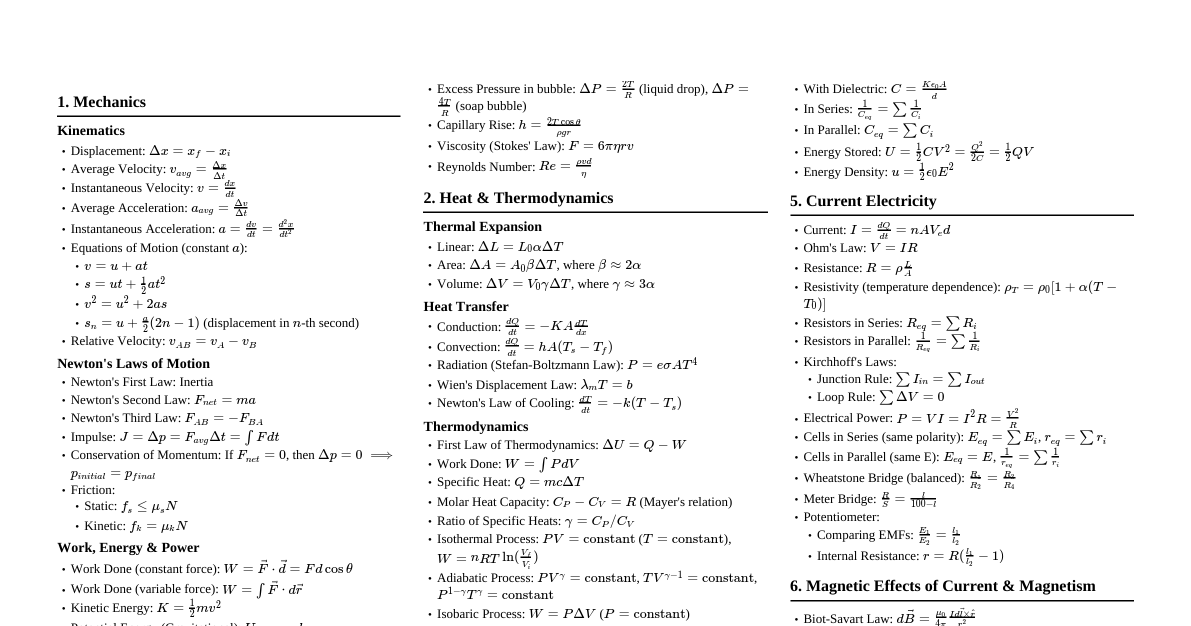
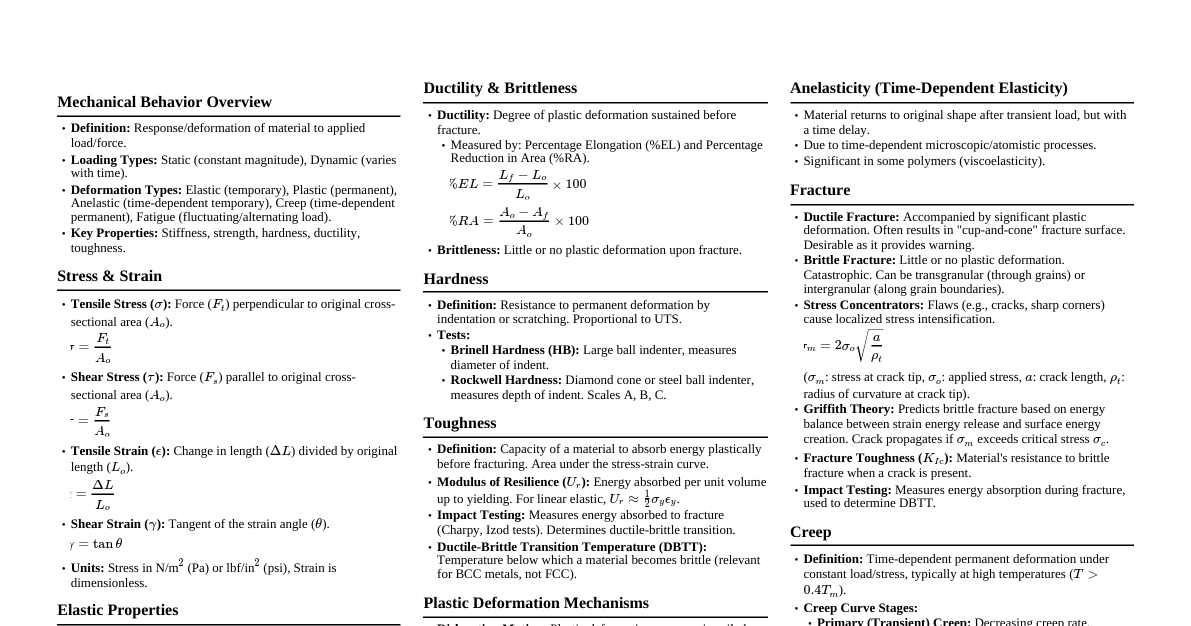
### Mechanical Properties of Solids #### 1. Elasticity & Plasticity - **Elasticity:** Property of a body to regain its original configuration after removal of deforming forces. - **Plasticity:** Property of a body to not regain its original configuration after removal of deforming forces. - **Elastic Limit:** Maximum stress a material can withstand without permanent deformation. #### 2. Stress & Strain - **Stress ($\sigma$):** Restoring force per unit area. - $\sigma = \frac{F}{A}$ (Unit: N/m$^2$ or Pascal (Pa)) - Types: Longitudinal (tensile/compressive), Tangential (shearing), Volumetric. - **Strain ($\epsilon$):** Ratio of change in configuration to original configuration. - $\epsilon = \frac{\Delta L}{L}$ (Longitudinal), $\epsilon_s = \frac{\Delta x}{L} = \tan\theta \approx \theta$ (Shearing), $\epsilon_v = \frac{\Delta V}{V}$ (Volumetric). - Unit: Dimensionless. #### 3. Hooke's Law - Within the elastic limit, stress is directly proportional to strain. - $\sigma \propto \epsilon \implies \sigma = E\epsilon$ - **Modulus of Elasticity (E):** Constant of proportionality. #### 4. Types of Moduli of Elasticity - **Young's Modulus (Y):** For longitudinal stress and strain. - $Y = \frac{\text{Longitudinal Stress}}{\text{Longitudinal Strain}} = \frac{F/A}{\Delta L/L}$ (Unit: Pa) - **Bulk Modulus (B):** For volumetric stress and strain. - $B = \frac{\text{Volumetric Stress}}{\text{Volumetric Strain}} = \frac{-P}{\Delta V/V}$ (Unit: Pa) - **Compressibility (K):** $K = \frac{1}{B}$ - **Shear Modulus (G) or Modulus of Rigidity:** For tangential stress and strain. - $G = \frac{\text{Tangential Stress}}{\text{Shearing Strain}} = \frac{F/A}{\theta}$ (Unit: Pa) #### 5. Poisson's Ratio ($\nu$) - Ratio of lateral strain to longitudinal strain. - $\nu = \frac{\text{Lateral Strain}}{\text{Longitudinal Strain}} = \frac{-\Delta d/d}{\Delta L/L}$ - Range: $0 \le \nu \le 0.5$ (theoretical), $-1 \le \nu \le 0.5$ (practical). #### 6. Stress-Strain Curve - **Proportional Limit:** Stress is proportional to strain. - **Elastic Limit:** Material returns to original state. - **Yield Point:** Beyond this, permanent deformation occurs. - **Tensile Strength:** Maximum stress before fracture. - **Fracture Point:** Material breaks. - **Ductile Materials:** Large plastic range (e.g., copper, aluminum). - **Brittle Materials:** Small plastic range (e.g., cast iron, glass). - **Elastomers:** No plastic range, large elastic region (e.g., rubber). #### 7. Elastic Potential Energy - Energy stored per unit volume due to deformation. - $U = \frac{1}{2} \times \text{Stress} \times \text{Strain} = \frac{1}{2} Y (\text{Strain})^2 = \frac{1}{2Y} (\text{Stress})^2$ ### Mechanical Properties of Liquids (Fluids) #### 1. Pressure - **Pressure (P):** Force per unit area exerted by fluid. - $P = \frac{F}{A}$ (Unit: Pa) - **Pressure at Depth (h):** $P = P_0 + \rho gh$ (where $P_0$ is atmospheric pressure, $\rho$ is fluid density). - **Pascal's Law:** Pressure applied to an enclosed fluid is transmitted undiminished to every portion of the fluid and the walls of the containing vessel. - Hydraulic lift: $\frac{F_1}{A_1} = \frac{F_2}{A_2}$ #### 2. Buoyancy & Archimedes' Principle - **Buoyant Force ($F_B$):** Upward force exerted by a fluid on a submerged or partially submerged object. - $F_B = \text{Weight of displaced fluid} = \rho_{fluid} V_{submerged} g$ - **Apparent Weight:** $W_{apparent} = W_{actual} - F_B$ #### 3. Fluid Dynamics - Flow Types - **Streamline (Laminar) Flow:** Fluid particles follow smooth paths, no mixing. Velocity at any point is constant over time. - **Turbulent Flow:** Irregular, chaotic flow with eddies and vortices. - **Critical Velocity:** Velocity above which laminar flow becomes turbulent. - **Reynolds Number ($R_e$):** Predicts flow type. - $R_e = \frac{\rho v D}{\eta}$ ($D$ is characteristic length, $\eta$ is viscosity). - $R_e 3000$: Turbulent, $2000 90^\circ$): Liquid doesn't wet solid (e.g., mercury-glass). - **Capillarity:** Rise or fall of liquid in a narrow tube (capillary). - **Jurin's Law:** $h = \frac{2T\cos\theta}{\rho rg}$ (where $r$ is capillary radius). - **Excess Pressure inside a bubble/drop:** - Liquid drop: $\Delta P = \frac{2T}{R}$ - Soap bubble: $\Delta P = \frac{4T}{R}$ ### Thermal Properties of Matter #### 1. Temperature & Heat - **Temperature:** Measure of the hotness or coldness of a body. Average kinetic energy of molecules. - Scales: Celsius ($^\circ$C), Fahrenheit ($^\circ$F), Kelvin (K). - Conversion: $\frac{C}{5} = \frac{F-32}{9} = \frac{K-273.15}{5}$ - **Heat (Q):** Form of energy transferred due to temperature difference. Unit: Joule (J) or calorie (cal). - 1 calorie = 4.186 J. #### 2. Thermal Expansion - **Linear Expansion:** $\Delta L = L_0 \alpha \Delta T$ ($\alpha$: coefficient of linear expansion). - **Area Expansion:** $\Delta A = A_0 \beta \Delta T$ ($\beta = 2\alpha$: coefficient of area expansion). - **Volume Expansion:** $\Delta V = V_0 \gamma \Delta T$ ($\gamma = 3\alpha$: coefficient of volume expansion). - **Anomalous Expansion of Water:** Water contracts from $0^\circ$C to $4^\circ$C, then expands. Density is maximum at $4^\circ$C. #### 3. Heat Capacity & Specific Heat - **Heat Capacity (C):** Amount of heat required to raise the temperature of a substance by $1^\circ$C or 1 K. - $C = \frac{\Delta Q}{\Delta T}$ (Unit: J/K) - **Specific Heat Capacity (c):** Heat capacity per unit mass. - $c = \frac{C}{m} = \frac{1}{m}\frac{\Delta Q}{\Delta T}$ (Unit: J kg$^{-1}$ K$^{-1}$) - Heat absorbed/released: $Q = mc\Delta T$ - **Molar Specific Heat:** Heat capacity per mole. - $C_p$ (at constant pressure), $C_v$ (at constant volume). - **Mayer's Relation:** $C_p - C_v = R$ (for ideal gas). - **Ratio of Specific Heats ($\gamma$):** $\gamma = \frac{C_p}{C_v}$ #### 4. Latent Heat - **Latent Heat (L):** Heat absorbed or released during a phase change at constant temperature. - $Q = mL$ - **Latent Heat of Fusion ($L_f$):** For solid-liquid transition. - **Latent Heat of Vaporization ($L_v$):** For liquid-gas transition. #### 5. Heat Transfer Mechanisms ##### a. Conduction - Transfer of heat through direct contact without actual movement of matter. - **Fourier's Law:** $\frac{dQ}{dt} = -KA\frac{dT}{dx}$ (K: thermal conductivity). - **Thermal Resistance:** $R_T = \frac{L}{KA}$ ##### b. Convection - Transfer of heat by actual movement of fluid particles. - **Natural Convection:** Due to density differences. - **Forced Convection:** Due to external means (e.g., fan). ##### c. Radiation - Transfer of heat in the form of electromagnetic waves, no medium required. - **Stefan-Boltzmann Law:** Power radiated by a black body: $P = \sigma A T^4$ ($\sigma$: Stefan-Boltzmann constant). - For a body with emissivity $e$: $P = e\sigma A T^4$. - Net power radiated by a body at temperature T in surroundings $T_0$: $P_{net} = e\sigma A (T^4 - T_0^4)$. - **Wien's Displacement Law:** $\lambda_m T = b$ (b: Wien's constant). $\lambda_m$ is wavelength corresponding to maximum emission. - **Newton's Law of Cooling:** Rate of cooling is proportional to the temperature difference between the body and surroundings (for small differences). - $\frac{dT}{dt} \propto (T - T_0)$ #### 6. Black Body Radiation - An ideal body that absorbs all incident radiation and emits maximum possible radiation at any given temperature. - Its emissivity $e=1$.