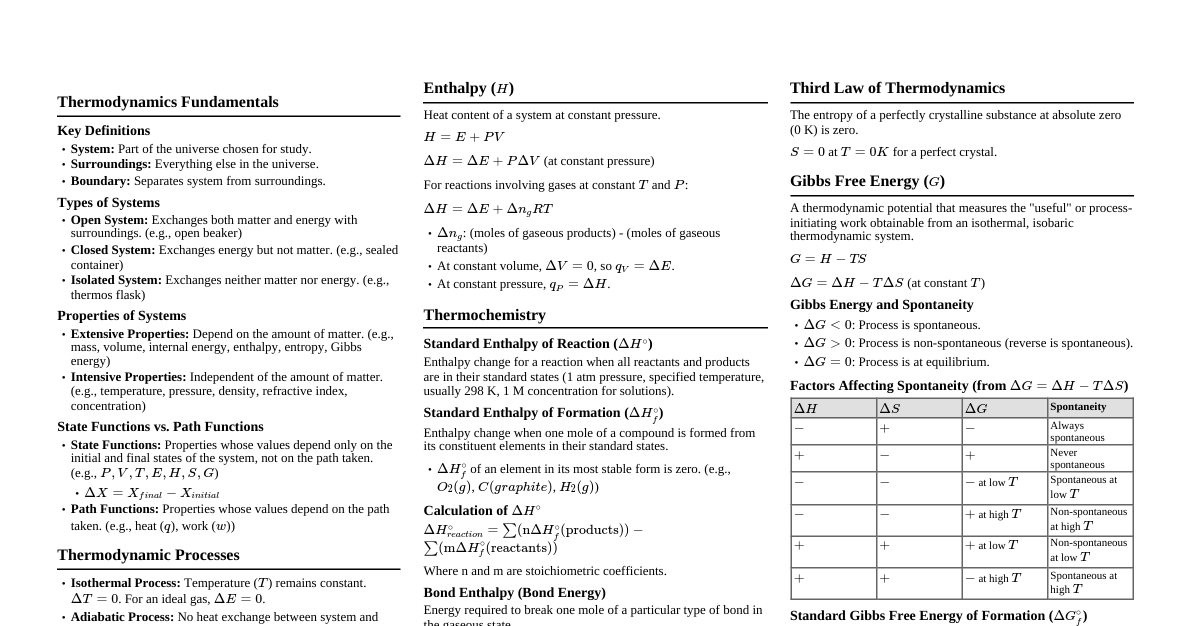
Introduction to Enthalpy ($H$) Enthalpy is a thermodynamic property representing the total heat content of a system at constant pressure. It's defined as $H = U + PV$, where $U$ is the internal energy, $P$ is pressure, and $V$ is volume. We usually measure changes in enthalpy, $\Delta H$, for chemical reactions. A negative $\Delta H$ indicates an exothermic reaction (releases heat), while a positive $\Delta H$ indicates an endothermic reaction (absorbs heat). Standard Enthalpy of Reaction ($\Delta H^\circ_{rxn}$) The enthalpy change for a reaction when all reactants and products are in their standard states (1 atm pressure for gases, 1 M concentration for solutions, pure substances for solids/liquids, usually at 298 K). Formula: $$ \Delta H^\circ_{rxn} = \sum n \Delta H^\circ_f (\text{products}) - \sum m \Delta H^\circ_f (\text{reactants}) $$ where $n$ and $m$ are stoichiometric coefficients. Example: Combustion of Methane CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) Given standard enthalpies of formation ($\Delta H^\circ_f$): CH₄(g): $-74.8 \text{ kJ/mol}$ O₂(g): $0 \text{ kJ/mol}$ (element in standard state) CO₂(g): $-393.5 \text{ kJ/mol}$ H₂O(l): $-285.8 \text{ kJ/mol}$ Calculation: $$ \Delta H^\circ_{rxn} = [1 \cdot (-393.5) + 2 \cdot (-285.8)] - [1 \cdot (-74.8) + 2 \cdot (0)] $$ $$ \Delta H^\circ_{rxn} = -965.1 + 74.8 = -890.3 \text{ kJ/mol} $$ Standard Enthalpy of Formation ($\Delta H^\circ_f$) The enthalpy change when one mole of a compound is formed from its constituent elements in their standard states. $\Delta H^\circ_f$ for an element in its standard state (e.g., O₂(g), C(s, graphite), H₂(g)) is $0 \text{ kJ/mol}$. Example: Formation of Water H₂(g) + ½O₂(g) → H₂O(l) The enthalpy change for this reaction is the standard enthalpy of formation of liquid water, $\Delta H^\circ_f [\text{H₂O(l)}] = -285.8 \text{ kJ/mol}$. Standard Enthalpy of Combustion ($\Delta H^\circ_c$) The enthalpy change when one mole of a substance undergoes complete combustion with oxygen under standard conditions. Example: Combustion of Ethanol C₂H₅OH(l) + 3O₂(g) → 2CO₂(g) + 3H₂O(l) The $\Delta H^\circ_{rxn}$ for this specific reaction, with one mole of ethanol, is the standard enthalpy of combustion of ethanol. Standard Enthalpy of Neutralization ($\Delta H^\circ_{neut}$) The enthalpy change when one mole of water is formed from the reaction of an acid and a base under standard conditions. Example: Strong Acid-Strong Base HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) For strong acid-strong base reactions, $\Delta H^\circ_{neut}$ is typically around $-57.3 \text{ kJ/mol}$ of water formed. H⁺(aq) + OH⁻(aq) → H₂O(l) Standard Enthalpy of Fusion ($\Delta H^\circ_{fus}$) The energy required to melt one mole of a solid substance into a liquid at its melting point and constant pressure. Example: Melting of Ice H₂O(s) → H₂O(l) $\Delta H^\circ_{fus} [\text{H₂O}] = +6.01 \text{ kJ/mol}$ at $0^\circ\text{C}$. This is an endothermic process. Standard Enthalpy of Vaporization ($\Delta H^\circ_{vap}$) The energy required to vaporize one mole of a liquid substance into a gas at its boiling point and constant pressure. Example: Boiling of Water H₂O(l) → H₂O(g) $\Delta H^\circ_{vap} [\text{H₂O}] = +40.7 \text{ kJ/mol}$ at $100^\circ\text{C}$. This is an endothermic process. Standard Enthalpy of Sublimation ($\Delta H^\circ_{sub}$) The energy required to convert one mole of a solid directly into a gas at constant pressure. Relationship: $$ \Delta H^\circ_{sub} = \Delta H^\circ_{fus} + \Delta H^\circ_{vap} $$ Example: Sublimation of Dry Ice CO₂(s) → CO₂(g) $\Delta H^\circ_{sub} [\text{CO₂}] = +25.2 \text{ kJ/mol}$ (at $-78.5^\circ\text{C}$). Standard Enthalpy of Solution ($\Delta H^\circ_{soln}$) The enthalpy change when one mole of a solute dissolves in a solvent to form a solution under standard conditions. This can be broken down into lattice energy and hydration energy. Example: Dissolution of NaCl NaCl(s) + H₂O(l) → Na⁺(aq) + Cl⁻(aq) $\Delta H^\circ_{soln} [\text{NaCl}] = +3.87 \text{ kJ/mol}$. Some dissolution processes are endothermic, others exothermic. Standard Enthalpy of Hydration ($\Delta H^\circ_{hyd}$) The enthalpy change when one mole of gaseous ions dissolves in sufficient water to form an infinitely dilute solution. This is always an exothermic process due to the strong ion-dipole interactions between ions and water molecules. Example: Hydration of Chloride Ion Cl⁻(g) + H₂O(l) → Cl⁻(aq) $\Delta H^\circ_{hyd} [\text{Cl⁻}] = -363 \text{ kJ/mol}$ Bond Enthalpy (Bond Energy) The average enthalpy change required to break one mole of a specific type of bond in the gaseous state. Breaking bonds is an endothermic process ($\Delta H > 0$). Forming bonds is an exothermic process ($\Delta H Formula (approximate): $$ \Delta H^\circ_{rxn} \approx \sum (\text{bond energies of bonds broken}) - \sum (\text{bond energies of bonds formed}) $$ Example: Hydrogenation of Ethene C₂H₄(g) + H₂(g) → C₂H₆(g) Bonds broken: 1 C=C, 1 H-H, 4 C-H Bonds formed: 1 C-C, 6 C-H Using average bond energies: Bond Energy (kJ/mol) C=C 614 C-C 348 C-H 413 H-H 436 Bonds broken: $(1 \cdot 614) + (1 \cdot 436) + (4 \cdot 413) = 614 + 436 + 1652 = 2702 \text{ kJ/mol}$ Bonds formed: $(1 \cdot 348) + (6 \cdot 413) = 348 + 2478 = 2826 \text{ kJ/mol}$ $$ \Delta H^\circ_{rxn} \approx 2702 - 2826 = -124 \text{ kJ/mol} $$ Hess's Law If a reaction can be expressed as the sum of a series of steps, then the enthalpy change for the overall reaction is the sum of the enthalpy changes for each step. This allows calculation of $\Delta H$ for reactions that are difficult to measure directly. Rules: If a reaction is reversed, the sign of $\Delta H$ is reversed. If the coefficients of a reaction are multiplied by a factor, $\Delta H$ is multiplied by the same factor. Example: Calculate $\Delta H$ for C(s) + O₂(g) → CO₂(g) Given: C(s) + ½O₂(g) → CO(g) ; $\Delta H_1 = -110.5 \text{ kJ}$ CO(g) + ½O₂(g) → CO₂(g) ; $\Delta H_2 = -283.0 \text{ kJ}$ Adding the two reactions directly gives the target reaction: C(s) + ½O₂(g) + CO(g) + ½O₂(g) → CO(g) + CO₂(g) Simplifying: C(s) + O₂(g) → CO₂(g) Therefore: $$ \Delta H_{rxn} = \Delta H_1 + \Delta H_2 = (-110.5) + (-283.0) = -393.5 \text{ kJ} $$ Entropy ($S$) Entropy is a measure of the disorder or randomness of a system. A positive change in entropy ($\Delta S > 0$) indicates an increase in disorder, while a negative change ($\Delta S Units for entropy are typically $\text{J/(mol·K)}$. Factors Affecting Entropy: Phase changes: $S_{\text{gas}} > S_{\text{liquid}} > S_{\text{solid}}$ Temperature: Increasing temperature generally increases entropy. Number of particles: More particles or moles of gas generally means higher entropy. Volume: Increasing volume (for gases) increases entropy. Complexity: More complex molecules tend to have higher entropy. Standard Molar Entropy ($S^\circ$) The absolute entropy of one mole of a substance in its standard state (1 atm, 298 K). Unlike enthalpy of formation, absolute entropies can be determined. Standard Entropy Change of Reaction ($\Delta S^\circ_{rxn}$) The change in entropy for a reaction under standard conditions. Formula: $$ \Delta S^\circ_{rxn} = \sum n S^\circ (\text{products}) - \sum m S^\circ (\text{reactants}) $$ where $n$ and $m$ are stoichiometric coefficients. Example: Formation of Ammonia N₂(g) + 3H₂(g) → 2NH₃(g) Given standard molar entropies ($S^\circ$): N₂(g): $191.6 \text{ J/(mol·K)}$ H₂(g): $130.7 \text{ J/(mol·K)}$ NH₃(g): $192.5 \text{ J/(mol·K)}$ Calculation: $$ \Delta S^\circ_{rxn} = [2 \cdot (192.5)] - [1 \cdot (191.6) + 3 \cdot (130.7)] $$ $$ \Delta S^\circ_{rxn} = [385.0] - [191.6 + 392.1] $$ $$ \Delta S^\circ_{rxn} = 385.0 - 583.7 = -198.7 \text{ J/(mol·K)} $$ The negative value indicates a decrease in disorder, consistent with forming fewer moles of gas (4 moles of gas reactants to 2 moles of gas products). Gibbs Free Energy ($G$) Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from an isothermal, isobaric thermodynamic system. It determines the spontaneity of a process. Formula: $$ G = H - TS $$ where $H$ is enthalpy, $T$ is temperature (in Kelvin), and $S$ is entropy. Standard Gibbs Free Energy Change of Reaction ($\Delta G^\circ_{rxn}$) The change in Gibbs free energy for a reaction under standard conditions (1 atm, 298 K). Formula: $$ \Delta G^\circ_{rxn} = \Delta H^\circ_{rxn} - T \Delta S^\circ_{rxn} $$ Alternatively, using standard free energies of formation: $$ \Delta G^\circ_{rxn} = \sum n \Delta G^\circ_f (\text{products}) - \sum m \Delta G^\circ_f (\text{reactants}) $$ where $\Delta G^\circ_f$ for an element in its standard state is $0 \text{ kJ/mol}$. Spontaneity Criteria (at constant T and P): If $\Delta G spontaneous (favors product formation). If $\Delta G > 0$: The reaction is non-spontaneous (favors reactant formation). If $\Delta G = 0$: The reaction is at equilibrium . Influence of $\Delta H$ and $\Delta S$ on Spontaneity: $\Delta H$ $\Delta S$ $\Delta G = \Delta H - T\Delta S$ Spontaneity $-$ (Exothermic) $+$ (Increased disorder) $-$ Spontaneous at all temperatures $+$ (Endothermic) $-$ (Decreased disorder) $+$ Non-spontaneous at all temperatures $-$ (Exothermic) $-$ (Decreased disorder) $-$ at low T, $+$ at high T Spontaneous at low temperatures $+$ (Endothermic) $+$ (Increased disorder) $+$ at low T, $-$ at high T Spontaneous at high temperatures Example: Formation of Ammonia (continued) From previous calculations: $\Delta H^\circ_{rxn} = -890.3 \text{ kJ/mol}$ (from combustion example, note: this refers to methane combustion, not ammonia formation. Let's use a value for ammonia formation for consistency ) Let's assume for ammonia formation: $\Delta H^\circ_{rxn} = -92.2 \text{ kJ/mol}$ $\Delta S^\circ_{rxn} = -198.7 \text{ J/(mol·K)} = -0.1987 \text{ kJ/(mol·K)}$ Standard temperature $T = 298 \text{ K}$ Calculation: $$ \Delta G^\circ_{rxn} = \Delta H^\circ_{rxn} - T \Delta S^\circ_{rxn} $$ $$ \Delta G^\circ_{rxn} = -92.2 \text{ kJ/mol} - (298 \text{ K})(-0.1987 \text{ kJ/(mol·K)}) $$ $$ \Delta G^\circ_{rxn} = -92.2 \text{ kJ/mol} + 59.2126 \text{ kJ/mol} $$ $$ \Delta G^\circ_{rxn} = -32.9874 \text{ kJ/mol} $$ Since $\Delta G^\circ_{rxn}$ is negative, the formation of ammonia is spontaneous under standard conditions. Gibbs Free Energy and Equilibrium Constant ($K$) The relationship between standard Gibbs free energy change and the equilibrium constant is: $$ \Delta G^\circ = -RT \ln K $$ where $R$ is the ideal gas constant ($8.314 \text{ J/(mol·K)}$) and $T$ is temperature in Kelvin. Non-Standard Conditions: For non-standard conditions, the Gibbs free energy change ($\Delta G$) is related to the reaction quotient ($Q$) by: $$ \Delta G = \Delta G^\circ + RT \ln Q $$ At equilibrium, $\Delta G = 0$ and $Q = K$, leading to $\Delta G^\circ = -RT \ln K$.