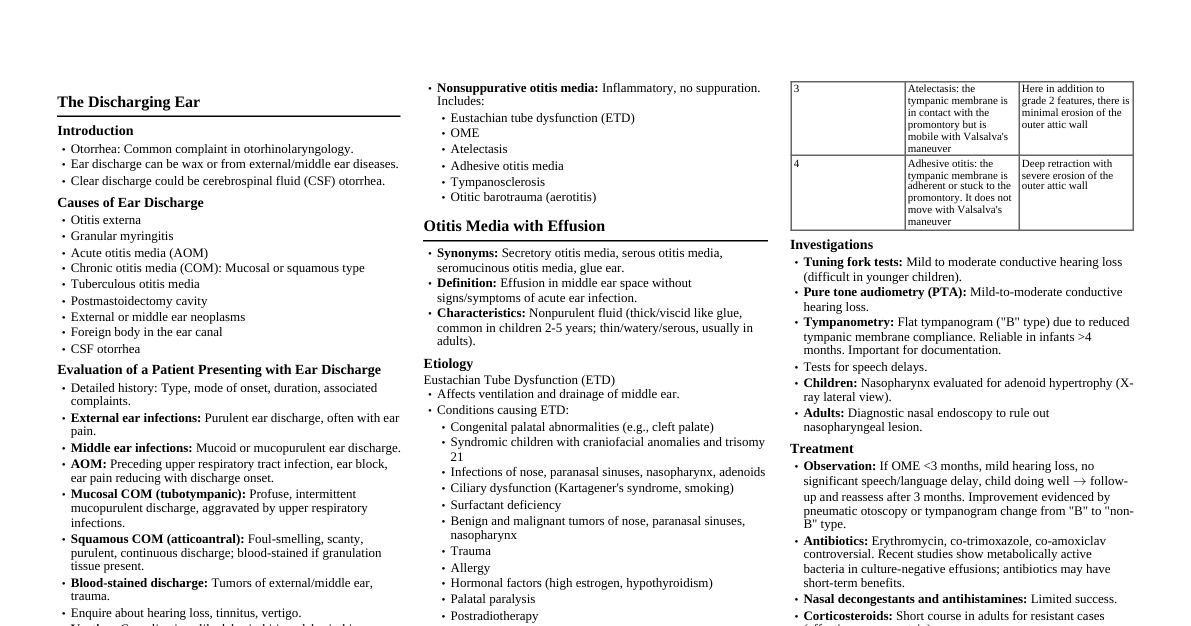
The Discharging Ear (Otorrhea) Ear discharge, or otorrhea, is a common complaint. It can be wax, or arise from diseases of the external or middle ear. Clear discharge may indicate cerebrospinal fluid (CSF) otorrhea. Causes of Ear Discharge Otitis externa Granular myringitis Acute otitis media (AOM) Chronic otitis media (COM): Mucosal or squamous type Tuberculous otitis media Postmastoidectomy cavity External or middle ear neoplasms Foreign body in the ear canal CSF otorrhea Evaluation of a Patient Presenting with Ear Discharge History: Type of discharge, mode of onset, duration, associated complaints (pain, hearing loss, tinnitus, vertigo). Purulent discharge: Often with ear pain, common in external ear infections. Mucoid/mucopurulent discharge: Characteristic of middle ear infections. AOM: Preceding upper respiratory tract infection, ear block, ear pain reducing with discharge onset. Mucosal COM (tubotympanic): Profuse, intermittent, mucoid/mucopurulent discharge, aggravated by URIs. Squamous COM (atticoantral): Foul-smelling, scanty, purulent, continuous discharge; may be blood-stained with granulation tissue. Blood-stained discharge: Seen in tumors of external/middle ear, and trauma. Vertigo: May indicate complications like labyrinthitis or labyrinthine fistula. Facial palsy with otorrhea: Possible complications of AOM, cholesteatoma, malignant otitis externa, temporal bone trauma, tuberculous otitis media, Ramsay Hunt syndrome. Petrositis: Otorrhea with deep-seated retro-orbital pain and diplopia. Watery discharge after head injury/mastoid surgery: Rule out CSF leak. Examination of the Ear Tragal tenderness: Otitis externa. Mastoid tenderness: Acute mastoiditis. Otoscopic examination: Identifies cause of discharge. Otitis externa: Inflamed and swollen external auditory canal skin. Otomycotic debris may be present. Malignant otitis externa: Granulation tissue in floor of external auditory canal at bony-cartilaginous junction. AOM: Perforation in anteroinferior quadrant with pulsatile ear discharge, congested pars tensa. Acute mastoiditis: Sagging of posterosuperior canal wall. Mucosal COM: Central perforation, margins formed by remnants of pars tensa. Squamous COM: Marginal, total, or attic perforation, or retraction pocket (posterosuperior quadrant or attic) with cholesteatoma. Recurrent/persistent discharge in post-mastoidectomy cavity: Due to residual disease, large cavity, deep recess, high facial ridge, inadequate saucerization/meatoplasty, exposed eustachian tube, ulceration/granulation/mucosalization of lining. Tuberculous otitis media: Multiple initial perforations coalescing into large perforation, pale granulations in middle ear. Investigations Otomicroscopy/otoendoscopy: Confirms diagnosis; swab for C&S. Ear cleaning (suction, dry mopping, wet mopping). Audiometry: Documents type and degree of hearing loss. Radiological investigations: HRCT scan: Temporal bone trauma, malignant otitis externa. Contrast-enhanced CT/MRI: Complications of otitis media or neoplastic lesions. Biopsy: For lesions not responding to medication, after imaging. Treatment of Ear Discharge Directed at treating the underlying cause and preventing complications. Otitis Media Introduction Inflammation or infection of the middle ear space. Spectrum: AOM, COM, otitis media with effusion (OME). Most inflammatory diseases linked to eustachian tube dysfunction. Classified as suppurative or nonsuppurative based on pus formation. Nonsuppurative otitis media: Inflammatory, no pus. Includes: Eustachian tube dysfunction OME Atelectasis Adhesive otitis media Tympanosclerosis Otitic barotrauma (aerotitis) Otitis Media with Effusion (OME) Synonyms: Secretory otitis media, serous otitis media, seromucinous otitis media, glue ear. Definition: Effusion in middle ear space without signs/symptoms of acute ear infection. Nonpurulent fluid (thick/viscid like glue, or thin/watery). More common in children (2-5 years), but can be seen in adults. Etiology Eustachian Tube Dysfunction Affects ventilation and drainage of the middle ear. Conditions causing it: Congenital palatal abnormalities (e.g., cleft palate). Syndromic children with craniofacial anomalies and trisomy 21. Infections of nose, paranasal sinuses, nasopharynx, and adenoids. Ciliary dysfunction (e.g., Kartagener's syndrome, smoking). Surfactant deficiency. Benign and malignant tumors of nose, paranasal sinuses, nasopharynx. Trauma. Allergy. Hormonal factors (high estrogen, hypothyroidism). Palatal paralysis. Postradiotherapy. Unresolved Acute Otitis Media (in Children) Impaired drainage and low-grade infection stimulate middle ear mucosa to secrete more fluid. Bacterial Biofilms Possible cause of middle ear effusion with eustachian tube dysfunction as secondary factor. Other Predisposing Factors Parental smoking. Stay in day care. Pearl A nasopharyngeal tumor should be suspected in adult patients presenting with features of unilateral OME. The tumor can block the eustachian tube opening at the nasopharyngeal end leading to OME. Clinical Features Symptoms Patients may be asymptomatic or report hearing loss with seasonal fluctuation. Conductive hearing loss in children (usually <40 dB), often leading to inattentiveness and affecting scholastic performance. Delayed speech and language development in younger children. Problems with balance or unexplained clumsiness; gross motor development delay. Children with craniofacial anomalies, syndromes, autism, or blindness are more adversely affected. Prior history of repeated URIs with otalgia, followed by reduced hearing. Adults: Ear block, tinnitus, decreased hearing, autophony; occasionally vertigo. Signs Otoscopic examination: Opaque with amber or gray colored tympanic membrane. Distorted or absent cone of light. Foreshortened handle of the malleus. Mild to severe retraction of tympanic membrane with prominent anterior and posterior malleolar folds. Reduced or absent mobility of tympanic membrane on air insufflation (Valsalva's maneuver or Siegelization). Air-fluid level or bubbles of air may be visible. Pneumatic otoscopy: Routinely done to aid diagnosis; movement of tympanic membrane during release of compressed bulb. Table 8.1 Grades of retractions of the tympanic membrane Grade Pars Tensa (Sade) Pars Flaccida (Tos and Paulsen) 1 Slight retraction of tympanic membrane over its annular fold Small attic dimple 2 Severe retraction of the tympanic membrane. It is draped over the long process of incus and incudostapedial joint Pars flaccida is retracted and in contact with the neck of the malleus 3 Atelectasis: the tympanic membrane is in contact with the promontory but is mobile with Valsalva's maneuver Here in addition to grade 2 features, there is minimal erosion of the outer attic wall 4 Adhesive otitis: the tympanic membrane is adherent or stuck to the promontory. It does not move with Valsalva's maneuver Deep retraction with severe erosion of the outer attic wall Tuning fork tests: Reveal mild to moderate conductive hearing loss (difficult in younger children). Investigations Pure tone audiometry (PTA): Mild-to-moderate conductive hearing loss. Tympanometry: Flat tympanogram ("B" type) due to reduced compliance. Reliable in infants >4 months. Important for documentation. Speech delays: Tests for speech delays. Nasopharynx evaluation: Children: X-ray for adenoid hypertrophy (lateral view). Adults: Diagnostic nasal endoscopy to rule out lesion. Treatment Observation: If OME Antibiotics (erythromycin, co-trimoxazole, co-amoxiclav): Controversial. Short-term benefits only. Nasal decongestants and antihistamines: Limited success. Corticosteroids: Short course in adults for resistant cases (effectiveness uncertain). Exercises: Valsalva's maneuver, Toynbee's maneuver, politzerization, Otovent autoinflation device (for children >3 years). Not for use with URI. Surgery: To restore middle ear ventilation, eliminate infection source, drain effusion. Myringotomy and grommet insertion: Common procedure. Aspiration of middle ear fluid via radial incision in anteroinferior quadrant; grommet (ventilation tube) inserted. Adenoidectomy: Removes obstruction to eustachian tube and infection focus. Eustachian tuboplasty (laser, microdebrider, balloon dilatation): Tried for persistent/recurrent OME. Steroids (topical nasal sprays like mometasone): Controversial, no clear evidence of effectiveness. Treat underlying cause: If OME due to nasopharyngeal carcinoma, treat tumor. Persistent OME may require myringotomy and grommet. Sequelae of OME Atelectasis of the middle ear. Adhesive otitis media. Atrophy of tympanic membrane or thinning (due to absence of middle fibrous layer, persistent negative middle ear pressure). Formation of a retraction pocket. Ossicular necrosis (commonly lenticular and long process of incus). Tympanosclerosis (hyaline degeneration and calcification of middle fibrous layer, thin avascular overlying mucosa). Cholesterol granuloma (cholesterol crystals surrounded by foreign body giant cells, fibrous connective tissue, inflammatory cells, blood vessels). Atelectasis Retraction of tympanic membrane onto promontory due to inadequate middle ear ventilation. Thin, transparent, atrophic tympanic membrane. Necrosis of ossicles with granulation formation. Adhesive Otitis Media Also called chronic middle ear catarrh. Fibrous adhesions in middle ear; tympanic membrane adherent to promontory and ossicles. Pearl Conditions causing conductive hearing loss with intact tympanic membrane: Otitis media with effusion. Tympanosclerosis. Ossicular discontinuity. Otosclerosis. Congenital cholesteatoma. Congenital fixation of malleus head or stapes. Persistent stapedial artery. Glomus tympanicum. Otitic Barotrauma (Aerotitis Media, Barotitis Media) Condition due to eustachian tube failure to maintain middle ear pressure at ambient atmospheric level. Symptoms manifest during deep sea diving, scuba diving, or descent from altitude in air travel. More common in non-pressurized aircraft. Predisposing Factors Poor eustachian tube function. Recent upper respiratory tract infection. Sleeping during flight. Deviated nasal septum, nasal polyps, allergic rhinitis (prevent equalization of pressure). Clinical Features Symptoms: Aural fullness, popping sensation, discomfort, pain. Asymptomatic once on ground level. Severe barotrauma: Temporary middle ear effusion or hemotympanum (due to negative pressure >90 mm Hg). Rapid pressure change: Tympanic membrane rupture. Inner ear involvement: Change in pressure can cause inner ear bleed or intralabyrinthine membrane rupture, leading to sensorineural hearing loss and vertigo. Diagnosis: By history. PTA: May show conductive hearing loss; rarely sensorineural or mixed. Pearl Signs of a retracted tympanic membrane: Dull, lusterless tympanic membrane. Absent or distorted cone of light. Apparent foreshortening of the malleus handle. Prominent lateral process of the malleus. Prominent anterior and posterior malleolar folds (sickle shaped). Reduced mobility of the tympanic membrane on Valsalva's maneuver, Toynbee's maneuver, or Siegelization. Treatment Mainly symptomatic: Decongestants and analgesics. Topical nasal decongestants: Xylometazoline or oxymetazoline (helps open eustachian tube). Oral decongestants: Phenylephrine. Eustachian tuboplasty: For frequent flyers. Long-standing ventilation tube: For professional/frequent flyers. Prevention Avoid traveling during URI. Correction of deviated nasal septum. Oral or topical nasal decongestants before travel. Acute Otitis Media (AOM) Introduction Acute infection of the middle ear mucoperiosteum by pyogenic organisms. Common in preschool children (prevalence in India 17-20%). Common in winter months, non-breastfed children. Recurrent AOM: >3 episodes in 6 months or 4-6 episodes in 1 year. Resistant AOM: Persists despite 3-5 days of antibiotics. Predisposing Factors Age: 6 months to 3 years. Allergies and genetic factors. Bottle feeding (especially in supine position) and pacifier use. Craniofacial anomalies: Cleft palate, Down's syndrome. Crowded living conditions, low socioeconomic status. Cystic fibrosis, primary ciliary dyskinesia. Anemia. Immunoglobulin deficiency (IgG, IgM). Exposure to smoke. Exposure to viral infections in day-care centers. Gastroesophageal reflux. Immunodeficiency. Infants: Wider, shorter, more horizontal eustachian tube; milk may enter middle ear if bottle-fed supine. Etiopathogenesis Viral URI: Involves middle ear via eustachian tube; releases inflammatory mediators, reduces ciliated cells, increases mucous production. Fluid in middle ear with impaired drainage leads to secondary bacterial infection. Children: Immature immune system. Infections (adenoiditis, tonsillitis, pharyngitis, rhinosinusitis): Spread to middle ear via eustachian tube. Forceful nose blowing/Valsalva's maneuver with rhinitis: May force infection into middle ear. Deep sea diving, barotrauma, swimming: Can cause AOM. Flying in unpressurized aircraft with rhinitis: Can cause AOM. Traumatic perforations: Facilitate infection spread from external ear. Rarely blood-borne infection. Causative Organisms Common: Streptococcus pneumoniae, Moraxella catarrhalis, Haemophilus influenza . Other: Staphylococcus aureus, S. haemolyticus . Rarely gram-negative: Proteus, Escherichia coli . Pearl Differential diagnosis of a blue drum: Hemotympanum. Glomus tympanicum. High jugular bulb with dehiscent floor of tympanic cavity. Cholesterol granuloma. Middle ear effusion. Clinical Features Usually preceded by viral URI. Rapid onset of ear pain (more severe at night). Infants: Irritability, intense crying, poor feeding. Toddlers: Clutch/rub ear, cry, sleep affected. Disease course described in 5 stages (Table 8.2). Table 8.2 Five stages based on the course of the disease Stage Symptoms Signs Stage of tubal occlusion (catarrhal stage) Blocking sensation/fullness in the ear, mild ear pain, mild deafness, fever and malaise Retracted tympanic membrane with loss of light reflex, tuning fork tests show conductive hearing loss Stage of presuppuration Severe throbbing ear pain (worsens during sleep), tinnitus (bubbling sounds), deafness (unnoticed), fever and malaise (patient looks toxic) Congested tympanic membrane with prominent blood vessels in pars tensa (cartwheel appearance), congestion of pars flaccida (epitympanitis), tuning fork tests show conductive hearing loss Stage of suppuration Excruciating ear pain, deafness, fever and other constitutional symptoms Congested and bulged tympanic membrane with loss of landmarks, yellow nipple (impending rupture), tenderness over suprameatal triangle (mastoiditis) Stage of resolution Blood-stained mucopurulent ear discharge, fever, ear pain, and constitutional symptoms subside Perforation in anteroinferior quadrant of pars tensa with blood-stained mucopurulent ear discharge (pulsatile, light house sign), conductive hearing loss Pearl Excessive crying in children can lead to congestion of the tympanic membrane, which needs to be differentiated from AOM. The tympanic membrane will not be edematous and light reflex will be present in a crying child. Stage of Complications Continuing inflammation (untreated/inadequately treated) leads to complications, especially with reduced host immunity or high organism virulence. Infection spread to mastoid air cell system: Acute mastoiditis, abscess. Other complications: Labyrinthitis, petrositis, facial paralysis, lateral sinus thrombophlebitis, meningitis, extradural abscess, brain abscess. Management Diagnosis: Clinical. Investigations: C&S of ear discharge, complete blood picture, C-reactive protein, blood culture. CT/MRI of temporal bone/head only if complications suspected or failure to improve. Medical Treatment Catarrhal stage: Antibiotics usually not required, most cases resolve spontaneously. Topical (nasal) decongestants: Oxymetazoline (0.025-0.05%), xylometazoline (0.05-0.1%). Oral decongestants with/without antihistamines. May reduce nasal symptoms in rhinitis. Antibiotics for suppuration: Amoxicillin (40 mg/kg/d, 7-10 days). Beta-lactamase producing organisms: Amoxicillin with clavulanate, cefixime, cefuroxime. Penicillin allergy: Oral cephalosporins (cefdinir, cefuroxime, cefpodoxime) or macrolides (clarithromycin). Severe infection: Parenteral antibiotics (amoxicillin with clavulanate or ceftriaxone) for 24-48 hours, followed by oral antibiotics for 7 days. Topical antibiotic ear drops: Not for use with discharge or pinhole/small perforations (may not enter middle ear effectively). Aural toilet: Removes discharge. Analgesics and antipyretics: Ibuprofen and/or acetaminophen for pain/fever. Surgical Treatment For medical management failure or impending complications. Three types: diagnostic, therapeutic, prophylactic. Tympanocentesis: Diagnostic, aspirates middle ear fluid with needle. Sent for C&S. Relieves pressure, reduces pain. Myringotomy: Therapeutic, incision (curvilinear) in posteroinferior quadrant of tympanic membrane to drain and ventilate middle ear cleft. Performed for: Exudative stage: Bulging tympanic membrane with pus. Suppurative stage: Small perforation with inadequate drainage. Impending complications. Myringotomy and grommet insertion: For recurrent AOM. Sequelae of Acute Otitis Media Conductive hearing loss (failure of resolution). Sensorineural hearing loss (damage to inner ear via toxin absorption from round window). Persistent perforation (due to eustachian tube pathology, common in large kidney-shaped perforation or poorly pneumatized mastoid). Atelectasis of tympanic membrane (due to persistent eustachian tube pathology). Healing of perforation with scarring or tympanosclerosis. Healing with dimeric membrane formation (middle fibrous layer absent). Pearl Most central perforations of the tympanic membrane (pars tensa) are kidney shaped because the parts of the membrane with abundant blood supply are in the peripheral annular region and along the handle of the malleus. The region in between is relatively avascular. Acute Necrotizing Otitis Media Severe, virulent AOM with necrosis of tympanic cavity. Common in pre-antibiotic era. Rapid onset/progression, seen in children with influenza, typhoid, measles, scarlet fever. Immunocompromised/malnourished children at higher risk. Secondary infection by $\beta$-hemolytic streptococci causes necrosis of tympanic membrane, ossicular chain, middle ear mucosa, mastoid air cells. Characteristics: Profuse otorrhea, kidney-shaped or near-total perforation, moderate to severe conductive or mixed hearing loss. Treatment: Early antibiotics (10-14 days). Cortical mastoidectomy if medical treatment fails or complicated by acute mastoiditis. Healing: Fibrosis of tympanic membrane, ingrowth of squamous epithelium from external auditory meatus. Chronic Otitis Media (Chronic Suppurative Otitis Media) Types of Chronic Otitis Media Mucosal type or tubotympanic disease (TTD). Squamous type or atticoantral disease (AAD). Mucosal Type or Tubotympanic Disease Mainly involves anteroinferior middle ear cleft. Associated with pars tensa perforation and ear discharge. Perforation is central. Ear discharge usually mucoid (goblet cells in anteroinferior middle ear cleft), can be mucopurulent. Can be active or inactive. Active Disease Perforation of pars tensa with ear discharge and/or middle ear inflammation, granulation tissue, or polyps. Discharge present at examination. Inactive Disease Perforation of pars tensa, absence of ear discharge and/or middle ear inflammation or polyps. No discharge at examination. Note (previous nomenclature): CSOM-tubotympanic type had four stages: Active stage: Discharge and/or congested middle ear mucosa, granulation, or polyp. Quiescent stage: Perforation of tympanic membrane, no discharge (last discharge within 6 months). Inactive stage: Perforation, last discharge >6 months ago. Healed stage: In dry (non-discharging) ears, perforation heals in two layers (dime ric membrane), may be associated with tympanosclerosis and conductive hearing loss. Permanent perforation: Squamous epithelium lines edges, outer epithelial and inner mucosal layers contact (contact inhibition). Occurs with insufficient blood supply, connective tissue hyperproliferation, less growth factors. Squamous Type or Atticoantral Disease (AAD) Chronic inflammatory condition of middle ear cleft, usually involving posterosuperior mesotympanum, attic, and antrum. Associated with entrapment of keratinizing squamous epithelium (cholesteatoma) in middle ear space, which can erode and destroy adjacent temporal bone tissues. Also called unsafe type of CSOM due to site and potential for intratemporal/intracranial complications. Hallmark: Cholesteatoma or granulations. Higher risk of complications. Cholesteatoma Synonyms: Epidermosis or keratoma. Term coined by Johannes Mueller in 1838. Misnomer (not a tumor, no cholesterol). Also "skin in the wrong place." Sac lined by stratified squamous epithelium with central mass of keratin debris; bone-eroding properties. Components (Fig. 8.14): Matrix: Squamous epithelium (stratum basale, superficiale, corneum) resting on thin fibrous stroma (perimatrix). Central white mass: Keratin debris produced by matrix. Causes of Bone Destruction by Cholesteatoma: Hyperemic decalcification. Osteoclastic bone resorption: Due to enzymes (acid phosphatase, collagenase, acid proteases, proteolytic enzymes, leukotrienes, cytokines). Pressure necrosis. Types of Cholesteatoma (Flowchart 8.1) Congenital Cholesteatoma: Originates from embryonal inclusion of squamous epithelium (cell rests) in middle ear cleft/temporal bone. Sites: tympanic cavity, petrous apex, cerebellopontine angle. Presents as white mass behind intact tympanic membrane with conductive hearing loss. Diagnostic criteria: Intact tympanic membrane, no previous history of otitis media. Acquired Cholesteatoma: Primary acquired: Formed in attic (pars flaccida) or posterosuperior quadrant (pars tensa) retraction pocket. Little/no history of ear discharge. Secondary acquired: Occurs in marginal perforation (usually posterosuperior quadrant of pars tensa) or long-standing pars tensa perforation with epithelial growth from margins onto medial middle ear wall. Characterized by foul-smelling ear discharge. Granulation tissue/polyps may be seen. Tertiary acquired: Following trauma or tympanoplasty (graft cholesteatoma after onlay myringoplasty). Pathogenesis of Acquired Cholesteatoma Impaired eustachian tube function causes middle ear pressure fluctuations. Blocked eustachian tube leads to air absorption, negative middle ear pressure, and retraction of pars flaccida/tensa. Invagination or Retraction Pocket Theory (Wittmack): Primary mechanism. Retraction pocket in attic or posterosuperior pars tensa with canal wall erosion. Due to eustachian tube dysfunction/epitympanic dysventilation. Pocket deepens from negative pressure and inflammation. Sac loses self-cleansing, secondary infection of keratin matrix. Sac expands, resulting in attic perforation or medial progression. Epithelial Invasion/Migration Theory (Habermann): Keratinizing squamous epithelium from tympanic membrane/external auditory canal migrates into middle ear through pre-existing perforation. Cells migrate by contact guidance, stopping when encountering other epithelial surface (contact inhibition). Basal Cell Hyperplasia (Ruedi and Lange): Inflammatory stimulus causes basal cells of stratum corneum from keratinizing epithelium of pars flaccida to invade sub-epithelial space, forming attic cholesteatoma. Metaplasia (Wendt): Pluripotent middle ear epithelium stimulated by inflammation transforms into metaplastic stratified squamous epithelium with keratinization. Implantation Theory: Iatrogenic skin implantation into middle ear (tympanoplasty, myringotomy, grommet insertion), or entrapment of epithelium via fracture line after trauma. Expansion of Cholesteatoma Enters middle ear cleft, invades surrounding structures by following path of least resistance, then enzymatic bone destruction. Pars flaccida cholesteatoma: Originates in Prussak's space (below scutum, limited by tympanic membrane, malleus neck, lateral malleus ligament). Extends laterally to ossicular chain, into epitympanum. Pars tensa cholesteatoma: Begins posterosuperiorly, extends posteriorly to facial recess/tympanic sinus, and medially to ossicular chain. Clinical Features (Squamous COM) Symptoms Persistent, scanty, purulent, foul-smelling, painless ear discharge. Blood-stained with granulations or polyps. No relation to URI, usually not completely subsiding with medication. Hearing loss: Usually conductive. Normal hearing in limited pars flaccida disease. "Cholesteatoma hearer" if ossicular chain eroded but gap bridged by cholesteatoma mass. Progressive conductive or mixed hearing loss (ossicular involvement, inner ear involvement by bacterial toxins/ototoxic drugs, cochlea/lateral semicircular canal erosion). Ear pain: Due to otitis externa secondary to discharge irritation. Dull pain from cholesteatoma filling mastoid antrum. Tinnitus and giddiness. Complications: Sudden severe vertigo (erosion into lateral semicircular canal). Sudden severe deafness (erosion into cochlea). Paralyzed face (facial nerve involvement). Swelling around ear with pain (mastoiditis). Fever, neck stiffness, severe headache (meningitis). Headache, retro-orbital pain, diplopia (petrositis). Headache, blurred vision, projectile vomiting (intracranial complications). Signs Retraction pocket or invagination of tympanic membrane in attic or posterosuperior pars tensa. Perforation in attic (pars flaccida) or posterosuperior pars tensa with marginal involvement. Attic perforation may be hidden by dried discharge/crusts. Attic disease can erode lateral attic wall (auto-atticotomy cavity) or extend into antrum (auto-mastoidectomy cavity). Crusts over pars flaccida should be removed. Even with pars tensa (central) perforation, pars flaccida examined to rule out double pathology. Total perforation involves fibrous annulus. Cholesteatoma debris or flakes in retraction pocket or other middle ear cleft parts. Granulation tissue in pars flaccida, posterosuperior pars tensa, posterosuperior deep meatus. During mastoidectomy, granulation in middle ear/mastoid. Perforation in pars tensa with ingrowing epithelium (velvety appearance). Aural polyp filling external auditory meatus. Erosion of posterior canal wall. Tuning fork tests: Suggestive of conductive or mixed hearing loss. Fistula sign: Positive with labyrinthine fistula (more common in lateral semicircular canal). Facial weakness: If facial paralysis. Types of Perforations of the Tympanic Membrane Tympanic membrane comprises pars tensa and pars flaccida. Pars tensa divided into four quadrants by vertical line along malleus handle and horizontal line at umbo. Perforations characterized by site, type, and annulus involvement. Perforation of Pars Tensa Central Perforation: Margins formed by remnants of pars tensa. Safe perforation. Can be: Small: Involving one quadrant or Medium: Involving two quadrants or 10-40% pars tensa. Large: Involving three or more quadrants or >40% pars tensa. Subtotal: All four quadrants, reaching but not involving fibrous annulus. Malleus handle may be skeletonized. Marginal Perforation: In pars tensa (commonly posterosuperior quadrant), erodes annulus, one margin formed by sulcus tympanicus. Unsafe perforation. Total Perforation: Complete perforation of pars tensa, margins formed by bony annulus. Unsafe perforation. Traumatic Perforation Usually occurs in posteroinferior quadrant, ragged edges with blood clots. Caused by trauma (slap, foreign body, iatrogenic, blast injury). Can affect ossicular chain (conductive) and inner ear (sensorineural). Clinical Features: Pain, bleeding, tinnitus (subsides). Vertigo. Perforation in posteroinferior quadrant (due to EAC curvature), ragged edges with blood clots. Blood in canal/over perforation. Tuning fork tests for initial hearing assessment. Treatment: Reassurance, wait-and-watch, regular follow-up. PTA to document hearing. Aural hygiene (prevent water entry, avoid earbuds). Margins approximated under vision. Topical ear drops not used. For contaminated injuries: cleaning, oral antibiotics. Most heal spontaneously. Myringoplasty if persistent. Perforation of the Pars Flaccida (Attic Perforation) Seen in pars flaccida. Associated with cholesteatoma. Unsafe perforation, seen in squamous COM or AAD. Chronic Otitis Media–Mucosal Type or Chronic Suppurative Otitis Media–TTD Definition Chronic inflammatory condition of middle ear cleft (eustachian tube, tympanic cavity, aditus, antrum, mastoid air cells) with permanent pars tensa perforation and intermittent otorrhea/hearing loss. Also known as CSOM-tubotympanic type, safe ear, benign type of CSOM. Inflammation leads to profuse discharge from mucous glands/goblet cells. No important structures involved, less chance of complications. Pearl A central perforation of the pars tensa is the hallmark of mucosal COM disease. Epidemiology Common cause of conductive hearing loss, especially in rural population (lack of health education, specialist accessibility). Poor socioeconomic status, improper nutrition contribute. Etiology Common in younger age group (school-going children). Both sexes, all age groups affected. Inadequately treated/untreated AOM leading to tympanic membrane perforation causes mucosal COM. Other causes: Traumatic perforation, grommet extrusion leading to permanent perforation (contact inhibition). Infections: Tonsils, adenoids, nasopharynx, nose, paranasal sinuses reach middle ear via eustachian tube, causing recurrent infection/ear discharge. Middle ear mucosa as shock organ to allergic reactions (ingested food, dust, hay) causing persistent otorrhea. Causative Organisms Most common: Pseudomonas aeruginosa . Other: Proteus, E. coli, S. pneumoniae, S. aureus . Bacterial biofilms play major role in chronic ear infections. Pearl Biofilms represent a form of survival mechanism for bacteria. Organisms enveloped in extracellular polysaccharide matrix. Difficult for antibiotics to penetrate, cause antibiotic resistance. Clinical Features (Mucosal COM) Symptoms Mucoid, non-foul-smelling, painless, non-blood-stained ear discharge. May be profuse, intermittent. Aggravated by URIs, cold climate, water entry. Reduces with medication. Causes for intermittent/persistent discharge: Anteriorly: Sinonasal infection, nasopharyngeal infection (adenoids), URI allergy. Laterally: Water entry, foreign body, cotton buds. Posteriorly: Mastoid reservoir of infection. Middle ear: Resistant organisms (rule out tuberculous otitis media). Bacterial biofilms: May cause persistent discharge (resistance to antibiotics). Poor patient compliance. Systemic causes: Diabetes, immunocompromised states. Hearing loss: Usually conductive, mild to severe. Round window shielding effect: Patient hears better with discharge (maintains phase differential). In dry ear, sound strikes round/oval windows simultaneously, canceling effect. Slowly progressive conductive hearing loss: Tympanosclerosis or fibrosis of ossicular chain. Mixed hearing loss: Due to bacterial toxins, cytokines, ototoxic drugs entering cochlea. Tinnitus: Present due to loss of ambient sound or toxins affecting inner ear. Otalgia: Not common. Pain usually due to secondary otitis externa. Signs Perforation of tympanic membrane: Usually central, may be anterior, inferior, or posterior to malleus handle. Varying size. Middle ear mucosa: Pale pink or moist (inactive) or congested/edematous (active). Polyp may be seen. Otomycosis in EAC (secondary to discharge or overuse of quinolone/steroid drops). Polyp: Pale, smooth, glistening mass of edematous mucosa protruding through perforation into EAC. May be congested/covered in discharge in active infection. Ossicular chain: Usually intact/normal. In long-standing cases, erosion (commonly long process of incus). Tympanosclerosis: White chalky deposits over tympanic membrane, ossicles, promontory, tendons, joints. Causes conductive hearing loss. External auditory canal: Filled with discharge, dried discharge, otomycosis debris. May be edematous due to secondary otitis externa. Investigations Pure Tone Audiometry: Assesses degree/type of hearing loss. Documents hearing loss for comparison with postoperative status. Suspect ossicular chain involvement (discontinuity or fixity) if air-bone gap >40 dB. Patch Test: Performed if air-bone gap >40 dB. Cigarette paper/thin filter paper covers perforation. Repeat PTA. Improvement suggests myringoplasty, no change suggests ossicular fixity or improper test. Culture and Sensitivity of Ear Discharge: Organisms: P. aeruginosa (commonest), Proteus, E. coli, S. aureus, Bacteroides , anaerobic streptococci. Swab taken from middle ear after cleaning EAC. X-Ray of the Mastoid: Prior to planned mastoidectomy. Gives info on mastoid type, dural/sinus plate levels. Chronic middle ear disease often shows secondary sclerotic mastoid. High-Resolution CT Scan of Temporal Bone: If mastoid reservoir of infection suspected. For intracranial pathology in mucosal disease, contrast-enhanced MRI. Diagnostic Nasal Endoscopy: If nasal/paranasal sinus disease symptoms present (source of infection). Treatment Aim: Eliminate infection, recreate barrier (tympanic cavity/EAC), restore hearing. Medical Management Aural Toilet: Removal of discharge/debris from EAC. Dry mopping (cotton on Jobson-Horne probe) or suction. Wet mopping (saline-soaked cotton) for dried discharge/ear drops. Topical Ear Drops: In actively discharging ear (3-4 times/day for 2 weeks). High drug concentration, low MIC. Combined with steroids. Common drops: Quinolones (ciprofloxacin, ofloxacin) with dexamethasone. 1.5% acetic acid: Creates acidic medium, helps eliminate Pseudomonas infection. Instillation: Patient supine, affected ear upward. Instill drops, tragal pumping. Stop if ear dries. Caution: May cause local allergy, skin maceration, fungal growth, resistance. Ototoxic drugs: Avoid. Systemic Antibiotics: If topical drops fail or cannot reach infected areas (mastoid air cells). If infection source outside middle ear cleft (tonsillitis, adenoiditis, rhinosinusitis). C&S required. Precautions: Avoid water entry (cotton with Vaseline/sterile ear plugs), swimming. Avoid forceful nose blowing. Discourage earbuds. Surgical Treatment For medical management failure or impending complications. Removal of Aural Polyps or Granulation Tissue: Prior to medical therapy, to facilitate antibiotic reach. Remove carefully, do not avulse (risk stapes, facial nerve, horizontal semicircular canal damage, giddiness, SNHL, facial nerve palsy, labyrinthitis). Cortical Mastoidectomy: For mastoid reservoir of infection. Some advocate for eustachian tube dysfunction (mastoid acts as buffer). Reconstructive Surgery: To close tympanic membrane defect, improve hearing. Myringoplasty: Surgical repair of tympanic membrane. Tympanoplasty: Eradicates disease from middle ear, reconstructs hearing mechanism. Chronic Otitis Media–Squamous Type or Chronic Suppurative Otitis Media–AAD Chronic inflammatory condition of middle ear cleft, usually involving posterosuperior mesotympanum, attic, and antrum. Associated with entrapment of keratinizing squamous epithelium (cholesteatoma) in middle ear space, which can erode and destroy adjacent tissues in the temporal bone. Also called unsafe type of CSOM due to site and potential for intratemporal/intracranial complications. Hallmark: Cholesteatoma or granulations. Higher risk of complications. Cholesteatoma Synonyms: Epidermosis or keratoma. Term coined by Johannes Mueller in 1838. Misnomer (not a tumor, no cholesterol). Also "skin in the wrong place." Sac lined by stratified squamous epithelium with central mass of keratin debris; bone-eroding properties. Components (Fig. 8.14): Matrix: Squamous epithelium (stratum basale, superficiale, corneum) resting on thin fibrous stroma (perimatrix). Central white mass: Keratin debris produced by matrix. Causes of Bone Destruction by Cholesteatoma: Hyperemic decalcification. Osteoclastic bone resorption: Due to enzymes (acid phosphatase, collagenase, acid proteases, proteolytic enzymes, leukotrienes, cytokines). Pressure necrosis. Types of Cholesteatoma (Flowchart 8.1) Congenital Cholesteatoma: Originates from embryonal inclusion of squamous epithelium (cell rests) in middle ear cleft/temporal bone. Sites: tympanic cavity, petrous apex, cerebellopontine angle. Presents as white mass behind intact tympanic membrane with conductive hearing loss. Diagnostic criteria: Intact tympanic membrane, no previous history of otitis media. Acquired Cholesteatoma: Primary acquired: Formed in attic (pars flaccida) or posterosuperior quadrant (pars tensa) retraction pocket. Little/no history of ear discharge. Secondary acquired: Occurs in marginal perforation (usually posterosuperior quadrant of pars tensa) or long-standing pars tensa perforation with epithelial growth from margins onto medial middle ear wall. Characterized by foul-smelling ear discharge. Granulation tissue/polyps may be seen. Tertiary acquired: Following trauma or tympanoplasty (graft cholesteatoma after onlay myringoplasty). Pathogenesis of Acquired Cholesteatoma Impaired eustachian tube function causes middle ear pressure fluctuations. Blocked eustachian tube leads to air absorption, negative middle ear pressure, and retraction of pars flaccida/tensa. Invagination or Retraction Pocket Theory (Wittmack): Primary mechanism. Retraction pocket in attic or posterosuperior pars tensa with canal wall erosion. Due to eustachian tube dysfunction/epitympanic dysventilation. Pocket deepens from negative pressure and inflammation. Sac loses self-cleansing, secondary infection of keratin matrix. Sac expands, resulting in attic perforation or medial progression. Epithelial Invasion/Migration Theory (Habermann): Keratinizing squamous epithelium from tympanic membrane/external auditory canal migrates into middle ear through pre-existing perforation. Cells migrate by contact guidance, stopping when encountering other epithelial surface (contact inhibition). Basal Cell Hyperplasia (Ruedi and Lange): Inflammatory stimulus causes basal cells of stratum corneum from keratinizing epithelium of pars flaccida to invade sub-epithelial space, forming attic cholesteatoma. Metaplasia (Wendt): Pluripotent middle ear epithelium stimulated by inflammation transforms into metaplastic stratified squamous epithelium with keratinization. Implantation Theory: Iatrogenic skin implantation into middle ear (tympanoplasty, myringotomy, grommet insertion), or entrapment of epithelium via fracture line after trauma. Expansion of Cholesteatoma Enters middle ear cleft, invades surrounding structures by following path of least resistance, then enzymatic bone destruction. Pars flaccida cholesteatoma: Originates in Prussak's space (below scutum, limited by tympanic membrane, malleus neck, lateral malleus ligament). Extends laterally to ossicular chain, into epitympanum. Pars tensa cholesteatoma: Begins posterosuperiorly, extends posteriorly to facial recess/tympanic sinus, and medially to ossicular chain. Clinical Features (Squamous COM) Symptoms Persistent, scanty, purulent, foul-smelling, painless ear discharge. Blood-stained with granulations or polyps. No relation to URI, usually not completely subsiding with medication. Hearing loss: Usually conductive. Normal hearing in limited pars flaccida disease. "Cholesteatoma hearer" if ossicular chain eroded but gap bridged by cholesteatoma mass. Progressive conductive or mixed hearing loss (ossicular involvement, inner ear involvement by bacterial toxins/ototoxic drugs, cochlea/lateral semicircular canal erosion). Ear pain: Due to otitis externa secondary to discharge irritation. Dull pain from cholesteatoma filling mastoid antrum. Tinnitus and giddiness. Complications: Sudden severe vertigo (erosion into lateral semicircular canal). Sudden severe deafness (erosion into cochlea). Paralyzed face (facial nerve involvement). Swelling around ear with pain (mastoiditis). Fever, neck stiffness, severe headache (meningitis). Headache, retro-orbital pain, diplopia (petrositis). Headache, blurred vision, projectile vomiting (intracranial complications). Signs Retraction pocket or invagination of tympanic membrane in attic or posterosuperior pars tensa. Perforation in attic (pars flaccida) or posterosuperior pars tensa with marginal involvement. Attic perforation may be hidden by dried discharge/crusts. Attic disease can erode lateral attic wall (auto-atticotomy cavity) or extend into antrum (auto-mastoidectomy cavity). Crusts over pars flaccida should be removed. Even with pars tensa (central) perforation, pars flaccida examined to rule out double pathology. Total perforation involves fibrous annulus. Cholesteatoma debris or flakes in retraction pocket or other middle ear cleft parts. Granulation tissue in pars flaccida, posterosuperior pars tensa, posterosuperior deep meatus. During mastoidectomy, granulation in middle ear/mastoid. Perforation in pars tensa with ingrowing epithelium (velvety appearance). Aural polyp filling external auditory meatus. Erosion of posterior canal wall. Tuning fork tests: Suggestive of conductive or mixed hearing loss. Fistula sign: Positive with labyrinthine fistula (more common in lateral semicircular canal). Facial weakness: If facial paralysis. Pearl When crusts are present over the pars flaccida, clean to look for underlying squamous disease. When there is a pars tensa perforation, examine the pars flaccida for any pathology as there may be signs of squamous COM. Investigations Microscopic examination: Confirms clinical findings. Pure tone audiogram (PTA): Documents presence, type, and degree of hearing loss. X-ray of the mastoid: Shows secondary sclerotic mastoid in long-standing disease. Cholesteatoma cavity (outer zone sclerosis, cotton wool appearance within, translucent zone between). HRCT scan of temporal bone: Extent of disease, ossicular chain status, mastoid type. Identifies erosion of sinus plate, dural plate, facial canal, lateral semicircular canal. Culture and sensitivity (C&S) of ear discharge: Less significant post-surgery. Organisms: P. aeruginosa (commonest), Proteus, E. coli, S. aureus, Bacteroides , anaerobic streptococci. Treatment Cholesteatoma is essentially surgical. Aim: Safe ear (eliminate cholesteatoma/chronic infection), conserve residual hearing, improve hearing where possible, achieve dry ear. Disease clearance: Canal wall up (atticotomy, atticoantrostomy for limited disease) or canal wall down (modified radical mastoidectomy for extensive disease). Tympanoplasty: For reconstruction of hearing mechanism. Conchomeatoplasty: If canal wall down procedure, to widen EAC for easy inspection/cleaning. Modified radical mastoidectomy: Canal wall down procedure to eradicate disease from middle ear cleft (exenteration of all accessible mastoid air cells, converting tympanic cavity, mastoid antrum, EAC into common cavity by removing posterior canal wall). Disease exteriorized through EAC by conchomeatoplasty. Complications of Otitis Media Occur when normal middle ear barriers are overcome, permitting infection spread. Distinguished from sequelae (tympanosclerosis, atelectasis, adhesive otitis media, ossicular erosion, perforation, cholesteatoma, hearing loss). Complications can occur with AOM or squamous COM. Less common in mucosal COM. Factors Favoring Complications: Bacteria with high virulence/antibiotic resistance. Patient factors: Age, immunosuppression, malnutrition, systemic diseases (tuberculosis, diabetes mellitus, malignancy). Inadequate treatment: Inadequate course/dose of antibiotic, wrong choice, lack of compliance. Poorly pneumatized mastoid. Pathophysiology and Routes of Spread Bone extension: Demineralized bone during acute infection or resorption by cholesteatoma/osteitis in chronic disease. Retrograde thrombophlebitis: Small veins through bone/dura to venous sinuses. Normal anatomical pathways: Oval and round window to inner ear. Cochlear or vestibular aqueducts. Dehiscence of thin bony covering of jugular bulb. Dehiscence of tegmen tympani. Dehiscence of temporal bone suture lines. Nonanatomical defects: Accidental/surgical trauma, neoplastic erosion. Other surgical defects: Vestibular opening (stapedectomy), lateral semicircular canal fenestration. Extension into brain tissue: Along periarteriolar Virchow-Robin spaces. Squamous COM can cause complications due to bone-eroding properties of cholesteatoma (Table 8.3). Table 8.3 Complications produced by squamous chronic otitis media Intratemporal Extratemporal Intracranial Acute coalescent mastoiditis Postauricular abscess Meningitis Masked mastoiditis Bezold's abscess (sternomastoid) Lateral sinus thrombophlebitis Acute petrositis Zygomatic abscess Extradural abscess Facial paralysis Luc's abscess (meatal) Subdural abscess Labyrinthitis Citelli's abscess (digastric) Brain abscess: temporal lobe/cerebellum Parapharyngeal abscess Otitic hydrocephalus Retropharyngeal abscess Intratemporal Complications Acute Mastoiditis Suppurative middle ear infections are associated with inflammation of mastoid air cells (proximity, continuity of lining mucosa). Mastoiditis with osteitis: Infection spreads to bony walls, breakdown of cells, acute coalescent mastoiditis (acute surgical mastoiditis). Predisposing Factors More common in children. Reduced host resistance: Malnutrition, immunosuppression, exanthematous fever, diabetes mellitus. Occurs in cellular mastoid. Causative Organisms S. pneumoniae, $\beta$-hemolytic streptococci, H. influenza . Occasionally gram-negative: Pseudomonas, Proteus, E. coli . Pathogenesis Flowchart 8.2 depicts the pathogenesis of acute mastoiditis. Hyperaemia and edema of mucoperiosteal lining of mastoid air cells. Thickened mucous membrane, impaired ciliary function and drainage of middle ear through eustachian tube. Obstruction/narrowing of aditus. Venous stasis and local acidosis. Dissolution of calcium from bony septae, increased osteoclastic activity in inflamed periosteum. Hyperaemic decalcification. Air cells coalesce into a larger cavity filled with purulent exudate and markedly thickened vascular mucoperiosteal granulations. Clinical Features Classically occurs 2 weeks after otorrhea onset. Symptoms similar to AOM (Table 8.4). Perforation of tympanic membrane, conductive hearing loss. Constitutional symptoms: Fever, malaise, toxic look. Table 8.4 Suggestive and definitive symptoms and signs Otorrhea persisting beyond 2 wk Presence of postauricular abscess Persistent or recurrent pain behind the ear Mastoid tenderness and swelling over the mastoid region-ironed out mastoid Erythema and edema over the mastoid tip Sagging of the posterosuperior meatal wall resulting from thickening of the periosteum of the bony external auditory meatus Investigations Complete blood count: Polymorphonuclear leukocytosis, raised ESR. Swab of discharge for C&S: From perforation after cleaning ear canal. X-ray of mastoids (lateral oblique Schuller's view): Diffuse clouding of air cells (fluid replacement, bony septal destruction). HRCT of temporal bone: Haziness of air cells, loss of bony trabeculae within mastoid process. Differential Diagnosis Postauricular suppurative lymphadenitis. Otitis externa: Furunculosis of EAC involving posterior canal wall. Treatment High-dose IV antibiotics: Based on C&S. Myringotomy: To facilitate pus drainage if no perforation. Cortical mastoidectomy: To exenterate accessible mastoid air cells if: Subperiosteal abscess. Sagging of posterosuperior meatal wall. Positive reservoir sign (persistent discharge after cleaning). No change/worsening after 48h antibiotics. Complications (facial nerve palsy, labyrinthitis, intracranial complications). Complications Subperiosteal abscess. Petrositis. Facial nerve paralysis. Labyrinthitis. Lateral sinus thrombophlebitis. Extradural abscess. Brain abscess. Otitic hydrocephalus. Subperiosteal Abscess Postauricular Abscess or Wilde's Abscess Hematogenous spread of infection through minute vascular channels in suprameatal triangle. Abscess displaces pinna forward, outward, downward. May be subperiosteal, subcutaneous, or open with discharge from mastoid cutaneous fistula. Zygomatic Abscess Infection in zygomatic air cells erodes through cortical bone at zygoma. Swelling above and in front of ear. Mear's or Luc's Abscess Pus breaks through bony wall of antrum/EAC, swelling visible in deep part of bony meatus. Citelli's Abscess Pus may travel along mastoid emissary vein or occipitotemporal suture, swelling posterior to mastoid. Also tracks along posterior belly of digastric muscle. Treatment IV antibiotics followed by incision/drainage and cortical mastoidectomy. Masked Mastoiditis (Latent Mastoiditis, Subacute Mastoiditis) Mastoiditis without draining ear or usual signs/symptoms. Slow destruction of mastoid air cells without acute signs. Seen in AOM modified by antibiotics. Clinical features: Mild pain over mastoid, persistent conductive hearing loss, thick tympanic membrane. X-ray shows extensive decalcification, coalescence of mastoid air cells. Treatment: Cortical mastoidectomy. Antibiotics based on C&S. Granulation tissue, dark gelatinous material seen during mastoidectomy. Petrositis Infection spreads from middle ear/mastoid to petrous part of temporal bone (anterior/posterior petrous apex). Etiology Associated with acute coalescent mastoiditis, latent mastoiditis, cholesteatoma. Organisms: Pneumococcus, H. influenza, $\beta$-hemolytic streptococci . Pathology Petrous apex related to abducens nerve, trigeminal ganglion. Pneumatized in 30%. Two groups of air cells lead to petrous apex: Posterosuperior tract: Starts in attic/antrum, runs around semicircular canals to petrous apex. Anteroinferior tract: Starts in hypotympanum, passes around eustachian tube/cochlea to petrous apex. Infection spreads along these tracts, can involve abducens nerve (Dorello's canal), trigeminal ganglion (Meckel's cave). Clinical Features Gradenigo's Syndrome: Lateral rectus palsy (paralysis of sixth cranial nerve) causing diplopia. Deep seated retro-orbital pain (trigeminal ganglion involvement). Persistent otorrhea. Other: Transient facial paresis, mild recurrent vertigo, low-grade intermittent fever, vomiting, headache. Investigations HRCT scan of temporal bone: Haziness in petrous apex cells. MRI: Better defines involvement of cells, abducens nerve, trigeminal ganglion. Delineates spread to cavernous sinus. Swab for C&S. Treatment High-dose antibiotics (5-7 days) followed by complete mastoidectomy. For persistent disease, surgical approach to petrous apex (transcochlear or translabyrinthine) to clear disease (affects hearing). Labyrinthitis Infection spread to inner ear. In AOM, bacterial toxins enter inner ear through oval/round windows. Other predisposing factors: Mondini malformation, labyrinthine fistula (detected with CT). Erosion of lateral semicircular canal by cholesteatoma leads to labyrinthine fistula. Clinical Features: Recurrent/transient vertigo, fluctuating SNHL. Nystagmus with positive fistula sign. Bony erosion of lateral semicircular canal on HRCT. Spread to subarachnoid space: Via cochlear aqueduct, causing meningitis. Meningitis can spread to labyrinth. Treatment: Antibiotics, labyrinthine sedatives. Steroids. Canal wall down mastoidectomy for cholesteatoma. Fistula covered by graft after complete cholesteatoma removal; otherwise, cholesteatoma sac everted to epithelialize. Facial Nerve Paralysis Can occur in AOM or with cholesteatoma. AOM: Within 2 weeks of onset, due to inflammatory edema (compression in fallopian canal or irritation of nerve) or osteitis. Exposure of dehiscent facial nerve segment (tympanic) to active infection. Cholesteatoma: Erosion of bony fallopian canal, exposing nerve to active infection, compression, or invasion. Management Facial palsy following AOM: Myringotomy (release pus), antibiotics. Mastoidectomy: If paralysis >2 weeks after AOM onset. If in COM. If paralysis fails to resolve after adequate management. ENoG: If >90% motor nerve fiber degeneration within 6 days of onset. Exploration of facial canal: Remove granulations. Cholesteatoma: Modified radical mastoidectomy with complete cholesteatoma removal (with/without facial nerve decompression). Intracranial Complications Extradural Abscess (Epidural Abscess) Collection of pus between bone and dura. Can occur with AOM or cholesteatoma. May precede brain abscess, coexist with sinus thrombophlebitis. Pathology Overlying dural bone destroyed by hyperemic decalcification (AOM) or enzymatic activity (cholesteatoma). Pus collection in epidural space. Dura covered with granulation, unhealthy, discolored. Abscess may be in middle cranial fossa, posterior cranial fossa, or perisinus (outside dura of lateral venous sinus). Clinical Features Some cases asymptomatic, discovered accidentally during mastoidectomy. Persistent headache (disappears with free pus flow from ear). Diagnosis Contrast-enhanced CT/MRI shows dural elevation. Treatment Antibiotic therapy (pre/post-surgery). Mastoidectomy: Remove overlying bone until healthy dura. If strong suspicion, remove tegmen tympani bone to check/evacuate pus. Observe for intracranial complications. Treat primary disease. Subdural Abscess Collection of pus in subdural space (between dura and arachnoid mater). Rare, fatal in pre-antibiotic era. Pathology Infection spread by bone/dura erosion or thrombophlebitic process. Pus against cerebral hemisphere creates pressure symptoms. Later loculated, can rupture. Clinical Features Sudden, severe, progressive symptoms. Severe throbbing headache, fever, vomiting, rapid deterioration points to subdural abscess. Due to meningeal irritation, cortical vein thrombophlebitis, raised CSF pressure. Meningeal irritation: Headache, high-grade fever, malaise, progressive drowsiness, neck rigidity, Kernig's sign. May be masked by prior antibiotics. Thrombophlebitis of cortical veins: Aphasia, contralateral hemiplegia, hemianopia, seizures. Raised CSF pressure: Papilledema, ptosis, dilated pupils (third cranial nerve involvement). Other cranial nerves may be involved. Diagnosis MRI superior to CT scan. Distinguishes epidural/subdural abscess. CT scan shows loculated subdural abscess. Lumbar puncture contraindicated (cerebellar tonsil herniation risk). Treatment Surgical emergency. Burr holes to drain abscess. High-dose broad-spectrum IV antibiotics. Mastoidectomy after abscess resolves, patient condition improves. Otogenic Brain Abscess Focal suppurative infection of brain parenchyma. ~50% in adults, 25% in children are otogenic. In adults, follows CSOM with cholesteatoma. In children, AOM. Cerebral abscesses (temporal lobe) commoner than cerebellar. Bimodal age distribution (pediatric, fourth decade). Route of Infection Direct extension of middle ear infection or retrograde thrombophlebitis of dural vessels. Often associated with extradural abscess. Cerebellar abscess: Direct extension through retrograde thrombophlebitis from sigmoid sinus (posterior mastoid wall). Bacteriology Both aerobic and anaerobic organisms. Polymicrobial cultures influenced by immune status. Aerobic: Pyogenic staphylococci, S. pneumoniae, S. haemolyticus, P. mirabilis, E. coli, P. aeruginosa . Anaerobic: Peptostreptococcus, B. fragilis . H. influenza rarely found. Pathogenesis Brain abscess develops in four stages: Invasion (initial encephalitis): Mild symptoms (headache, low-grade fever, malaise, drowsiness). Often unnoticed. Lasts 1-3 days. Localization (latent abscess): Pus localizes, capsule forms. Patients asymptomatic for days/weeks. Lasts 4-10 days. Enlargement (manifest abscess): Abscess enlarges, edema creates symptoms. Due to raised intracranial tension, cerebrum/cerebellum dysfunction. Lasts 10-13 days. Termination (rupture of abscess): Expanding abscess ruptures into ventricle/subarachnoid space, resulting in fatal meningitis. Clinical Features Often associated with extradural abscess, perisinus abscess, meningitis, sigmoid sinus thrombosis, labyrinthitis. Clinical picture can overlap. Toxic, drowsy. Dizziness, nystagmus, vomiting, ataxia (cerebellar abscess). Temporal abscess (seizures). Due to raised intracranial tension or affected brain area. Symptoms and Signs of Raised Intracranial Tension Headache: Severe, generalized, worse in morning. Nausea, projectile vomiting (more in cerebellar lesions). Altered consciousness: Lethargy to drowsiness, confusion, stupor, coma. Papilledema: Absent early, appears late (>2-3 weeks). Appears early in cerebellar abscess. Slow pulse, subnormal temperature. Localizing Features of a Temporal Lobe Abscess Nominal aphasia: (Dominant cerebral hemisphere, left in right-handed). Inability to name objects. Contralateral homonymous hemianopia: Pressure on optic radiations. Visual field defect (upper quadrant). Confrontation test. Contralateral motor paralysis: Upward spread (facial palsy, arm, leg paralysis) or inward spread (leg, arm, face paralysis). Epileptic fits: Small involuntary smacking movements (lips, tongue), generalized fits. Localizing Features of a Cerebellar Abscess Suboccipital headache with neck rigidity. Spontaneous nystagmus. Ipsilateral ataxia (staggering to side of lesion). Finger-nose test: Past pointing, intention tremor. Dysdiadochokinesia: Slow, irregular rapid pronation/supination. Investigation Contrast-enhanced CT scan: Confirms site/size of abscess. Associated complications (extradural abscess, sigmoid sinus thrombosis). Ring sign (hypodense area with edema). Temporal bone better visualized on CT. MRI: Detects subtle changes in brain parenchyma, spread of abscess. Lumbar puncture: Rise in pressure, increased protein, normal sugar, raised WBCs. Medical Treatment IV antibiotics: Chloramphenicol, penicillin, derivatives. Metronidazole (anaerobes). Aminoglycosides (pseudomonas, proteus). Changed per C&S. Dexamethasone (4 mg IV q6h) or 20% mannitol (0.5 g/kg): Reduces edema, intracranial tension. Surgical Treatment Life-saving neurosurgical intervention more important. Aspiration of pus for C&S. Repeat CT/MRI for resolution. Repeated aspirations. Excision if no size decrease or rapid enlargement. Otological Intervention Once neurologically stable, tympanomastoid surgery. Cholesteatoma requires modified radical mastoidectomy. Meningitis Inflammation of leptomeninges (pia, arachnoid) and CSF of subarachnoid space. Most common intracranial complication. Can occur in children/adults. Causes After AOM episode in children. Squamous COM disease. Temporal bone fracture causing CSF leak. Following middle ear/mastoid surgeries (rare). Route of Spread Infection from middle ear/mastoid reaches meninges via: Preformed pathways: Patent petrosquamous suture. Retrograde venous thrombophlebitis. Direct erosion of bone/dura. From labyrinthitis via cochlear aqueduct. Commonest organisms: H. influenzae, S. pneumoniae . Clinical Features Severity varies with extent of infection. Earliest symptoms: High-grade fever, chills, rigors. Throbbing headache, photophobia. Nausea, projectile vomiting. Irritability, restlessness. Infants may develop seizures. Signs (may be masked by antibiotics): Neck rigidity (earliest sign). Kernig's sign: Leg extension with thigh flexion on abdomen is painful. Brudzinski's sign: Neck flexion results in hip/knee flexion. Cranial nerve palsies, hemiplegia. Exaggerated deep tendon reflexes initially, sluggish/absent later. Investigations HRCT of temporal bone: Imaging modality of choice. Details bony erosion, congenital malformations, fistula. MRI: For inflammatory changes in brain, related complications. Lumbar puncture, CSF analysis: Establishes diagnosis. Cloudy/yellow (xanthochromic) CSF. Raised cell count (polymorphs predominance). Raised protein level. Low sugar, chlorides. C&S of CSF: Identifies organism, antibiotic sensitivity. Fundoscopy: Indistinct disc margins, choking of vessels. Ear swab: For C&S. Treatment Medical treatment takes precedence. Surgery indicated for failed medical therapy. Antibiotics: Crystalline penicillin, ampicillin, chloramphenicol, third-generation cephalosporins (IV for 7-10 days). Surgery when general condition improves. AOM: Myringotomy with/without cortical mastoidectomy. Cholesteatoma: Modified radical mastoidectomy. Lateral Sinus Thrombophlebitis (Sigmoid Sinus Thrombosis) Inflammation of inner wall of lateral sinus with thrombosis, later infection. Incidence decreased with antibiotics, but mortality remains high. Etiology Acute coalescent mastoiditis. Masked mastoiditis. Cholesteatoma. Common bacteriology: $\beta$-hemolytic Streptococcus, Pneumococcus, Bacillus, Proteus, Pseudomonas . Clinical Features Fever: Hectic, picket fence type with chills/rigors. One/more peaks/day, doesn't touch baseline (septic emboli release). Later, temperature fall with profuse sweating, sense of well-being. Headache: Mild (perisinus abscess). Severe with increased intracranial pressure. Deep-seated ear pain, ear discharge. Griesinger's sign: Mastoid emissary vein thrombosis leads to tenderness/edema posterior mastoid. Pathognomonic of sigmoid sinus thrombosis. Tenderness along internal jugular vein (in neck) from thrombosis. Enlarged, tender jugular lymph nodes. Anemia. Papilledema: Blurring of disc margins, retinal hemorrhages, dilated veins. Occurs when clots extend to superior sagittal sinus. Crowe-Beck test (Lily-Crowe sign): Pressure on opposite internal jugular vein causes retinal/supraorbital vein engorgement, subsides on release. Cavernous sinus thrombosis: Proptosis, ptosis, chemosis, ophthalmoplegia. Investigations Blood culture: Antibiotic change as per C&S. Peripheral smear: Rules out malarial parasites. C&S of ear discharge. Lumbar puncture: Normal CSF findings with rise in pressure. Rules out meningitis. Tobey-Ayer test: Records CSF pressure while compressing one/both jugular veins. Compression of affected side has no effect, opposite side causes rapid CSF pressure rise. Contrast-enhanced CT scan: "Delta sign" (empty triangular area with rim enhancement, central low density at sigmoid sinus). MRI (Gadolinium-enhanced): Thrombus appears as soft-tissue signal with vascular/bright dural walls. More sensitive than CT. MR angiography/venography: Demonstrates thrombus. Complications If untreated: Septicemia, meningitis, brain abscess. Septic thrombi cause pyogenic abscess in lungs, bones, joints, subcutaneous tissues. Thrombosis of jugular bulb/vein can involve cranial nerves IX-XI. Cavernous sinus thrombosis, otitic hydrocephalus. Treatment Antibiotic therapy: Initially crystalline penicillin (1M units q6h) to cover pyogenic organisms. For 10-14 days based on C&S. Mastoidectomy: Cortical or modified radical mastoidectomy for AOM/cholesteatoma. Bony sinus plate removed to expose dura, drain perisinus abscess. Ligation of internal jugular vein: If medical/surgical options fail (restricts thromboemboli spread). Blood transfusion: For anemia. Improve general well-being (supportive/nutritional). Anticoagulants: May help reduce clot propagation, promote sinus recanalization, reduce neurological deficits. Otitic Hydrocephalus Benign raised intracranial hypertension, no associated ventricular dilation. Rare complication with raised intracranial pressure and normal CSF. Seen in children/adolescents. Mechanism Increased CSF production. Obstruction to venous return (transverse/sigmoid sinus thrombosis). Reduced CSF absorption (arachnoid villi damage). Clinical Features Severe headache (usual complaint), nausea, vomiting. Traction on sixth cranial nerve causes diplopia. Increased intracranial pressure causes papilledema, optic atrophy, blurring of vision. Papilledema with patches of exudates/hemorrhages. Nystagmus. Diagnosis MRI: Evaluates venous sinuses. Lumbar puncture: CSF sterile, normal cell count/sugar/protein. Pressure >300 mm water (normal 70-120 mm). Treatment Medical: Reduce CSF pressure (corticosteroids, mannitol, acetazolamide). Surgical: Reduce CSF pressure, prevent optic atrophy/blindness (lumbar drain, repeated lumbar puncture, lumboperitoneal shunt). Mastoidectomy, decompression of sigmoid sinus. Optic sheath decompression. Points to Ponder OME: Common in children, hearing loss from fluid accumulation. Ear discharge: Common complaint, nature of discharge aids diagnosis. Mucoid/mucopurulent discharge in EAC implies tympanic membrane perforation (secreted by middle ear mucous glands/goblet cells). AOM: Common in infancy/early childhood due to eustachian tube anatomy (shorter, wider, more horizontal). COM: Mucosal or squamous. Both active/inactive. Old terminology (CSOM-TTD/AAD) now referred to as mucosal/squamous disease. CSOM-TTD: Safe type, not usually associated with complications. CSOM-AAD: Dangerous type, can cause complications due to cholesteatoma. Cholesteatoma: Sac lined by squamous epithelium with keratin mass. Bone-eroding properties, cause of complications. Complications of CSOM: Intratemporal, extratemporal, intracranial.