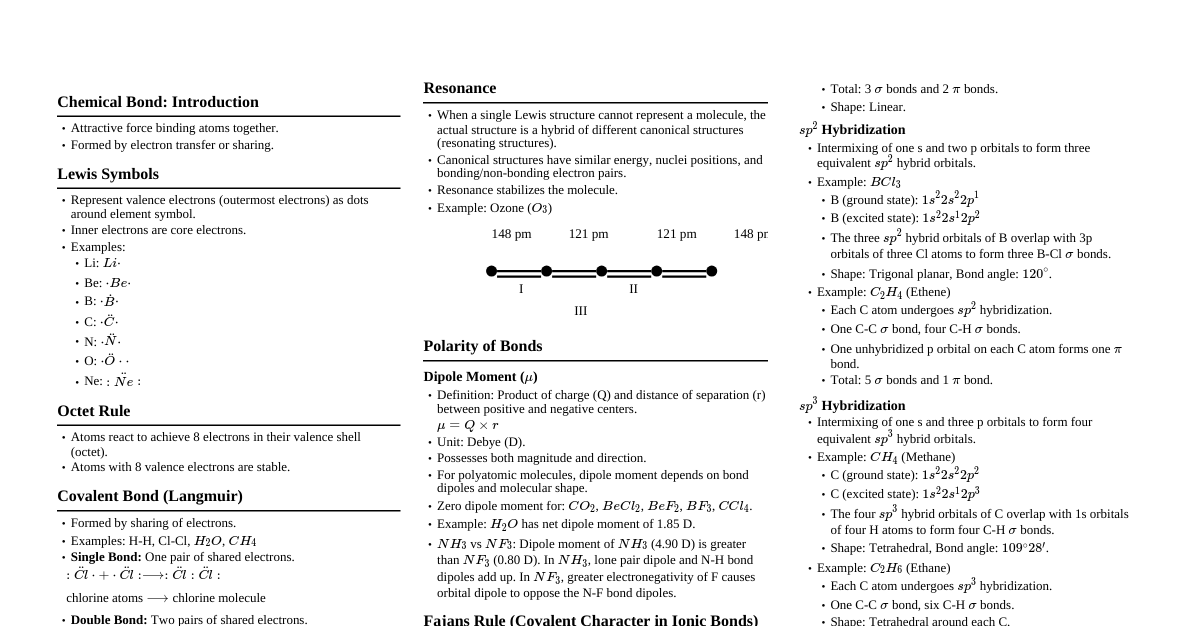
1. Discovery of Sub-atomic Particles 1.1 Discovery of Electron (Cathode Rays) Cathode rays start from the cathode and move towards the anode. They are not visible but glow when hitting fluorescent materials. Travel in straight lines in the absence of electric or magnetic fields. Consist of negatively charged particles called electrons . Characteristics of cathode rays (electrons) do not depend on the material of electrodes or the nature of the gas. 1.2 Charge to Mass Ratio of Electron ($e/m_e$) J.J. Thomson (1897) measured $e/m_e$ using cathode ray tube and perpendicular electric/magnetic fields. Value of $e/m_e = 1.758820 \times 10^{11} \text{ C kg}^{-1}$. Deflection depends on: Magnitude of negative charge: greater charge, greater deflection. Mass of particle: lighter particle, greater deflection. Strength of electric/magnetic field: stronger field, greater deflection. 1.3 Charge on the Electron ($e$) R.A. Millikan (1906-14) determined the charge using the oil drop experiment . Charge on electron $e = -1.6 \times 10^{-19} \text{ C}$. Accepted value: $-1.602176 \times 10^{-19} \text{ C}$. Mass of electron ($m_e$) calculated from $e/m_e$ ratio: $$m_e = \frac{e}{e/m_e} = \frac{1.602176 \times 10^{-19} \text{ C}}{1.758820 \times 10^{11} \text{ C kg}^{-1}} = 9.1094 \times 10^{-31} \text{ kg}$$ 1.4 Discovery of Protons (Canal Rays) Electrical discharge in modified cathode ray tubes led to canal rays (positively charged particles). Characteristics: Mass depends on the nature of gas. Charge to mass ratio depends on the gas. Some particles carry a multiple of the fundamental electrical charge unit. Behave opposite to electrons in electric/magnetic fields. Smallest, lightest positive ion from hydrogen was called proton (discovered by Rutherford in 1919). 1.5 Discovery of Neutrons Chadwick (1932) discovered neutrons by bombarding beryllium with $\alpha$-particles. Neutrons are electrically neutral particles with mass slightly greater than protons. 1.6 Properties of Fundamental Particles Name Symbol Absolute charge/C Relative charge Mass/kg Mass/u Approx. mass/u Electron e $-1.602176 \times 10^{-19}$ $-1$ $9.109382 \times 10^{-31}$ $0.00054$ $0$ Proton p $+1.602176 \times 10^{-19}$ $+1$ $1.6726216 \times 10^{-27}$ $1.00727$ $1$ Neutron n $0$ $0$ $1.674927 \times 10^{-27}$ $1.00867$ $1$ 2. Atomic Models 2.1 Thomson Model of Atom (Plum Pudding Model) Proposed by J.J. Thomson (1898). Atom is a sphere (radius $\approx 10^{-10}$ m) with uniformly distributed positive charge. Electrons are embedded in it to give the most stable electrostatic arrangement. Explained overall neutrality but failed to explain later experimental results. 2.2 Rutherford's Nuclear Model of Atom ($\alpha$-Particle Scattering Experiment) Rutherford and his students (Geiger and Marsden) bombarded thin gold foil with $\alpha$-particles. Observations: Most $\alpha$-particles passed undeflected. A small fraction deflected at small angles. Very few ($\approx 1$ in $20,000$) bounced back (deflected by $\approx 180^\circ$). Conclusions: Most of atom's space is empty. Positive charge concentrated in a very small volume called the nucleus . Electrons move around the nucleus in circular paths called orbits . Electrons and nucleus held together by electrostatic forces. Nucleus volume is negligibly small compared to atom volume (radius of atom $\approx 10^{-10}$ m, nucleus $\approx 10^{-15}$ m). Drawbacks: Could not explain stability of atom (electrons should spiral into nucleus). Said nothing about electron distribution or energies. 2.3 Atomic Number ($Z$) and Mass Number ($A$) Atomic Number ($Z$): Number of protons in the nucleus. $$Z = \text{Number of protons} = \text{Number of electrons (in neutral atom)}$$ Mass Number ($A$): Total number of nucleons (protons + neutrons). $$A = \text{Number of protons} (Z) + \text{Number of neutrons} (n)$$ Isotopes: Atoms with identical atomic number ($Z$) but different mass number ($A$) (different number of neutrons). E.g., Protium ($^1_1\text{H}$), Deuterium ($^2_1\text{D}$), Tritium ($^3_1\text{T}$). Isobars: Atoms with same mass number ($A$) but different atomic number ($Z$). E.g., $^{14}_6\text{C}$ and $^{14}_7\text{N}$. Chemical properties depend on electron number (which equals proton number). Neutrons have little effect. 3. Developments Leading to Bohr's Model 3.1 Dual Character of Electromagnetic Radiation Maxwell (1870) proposed that accelerating charged particles produce and transmit alternating electrical and magnetic fields as electromagnetic waves . Light is an electromagnetic wave with oscillating electric and magnetic components perpendicular to each other and to the direction of propagation. Electromagnetic waves do not require a medium and can travel in vacuum. They differ in wavelength ($\lambda$) or frequency ($\nu$), forming the electromagnetic spectrum . Speed of light ($c$) in vacuum is constant: $c = 3.0 \times 10^8 \text{ m s}^{-1}$. Relationship: $c = \nu \lambda$. Wavenumber ($\bar{\nu}$): Number of wavelengths per unit length. $\bar{\nu} = 1/\lambda$. 3.2 Particle Nature of Electromagnetic Radiation (Planck's Quantum Theory) Planck (1900) proposed that energy is emitted or absorbed in discrete quantities called quanta . Energy of a quantum: $E = h\nu$, where $h$ is Planck's constant ($6.626 \times 10^{-34} \text{ J s}$). Photoelectric Effect: Ejection of electrons from a metal surface when light strikes it (H. Hertz, 1887). Electrons ejected instantly. Number of electrons $\propto$ intensity of light. Requires a minimum frequency ( threshold frequency, $\nu_0$ ) for each metal. Kinetic energy of ejected electrons $KE \propto (\nu - \nu_0)$. Einstein (1905) explained the photoelectric effect using Planck's theory: $$h\nu = h\nu_0 + \frac{1}{2}mv^2$$ where $h\nu_0$ is the work function ($W_0$) , the minimum energy to eject an electron. Dual Behaviour of Light: Light exhibits both wave-like (interference, diffraction) and particle-like (photoelectric effect, black-body radiation) properties. 4. Bohr's Model for Hydrogen Atom Proposed by Niels Bohr (1913) using Planck's quantum concept. Postulates: Electrons move in fixed circular paths called orbits (stationary states). Electron energy in an orbit is constant. Energy changes only during transitions between orbits. Frequency of absorbed/emitted radiation during transition: $\nu = \frac{\Delta E}{h} = \frac{E_2 - E_1}{h}$ (Bohr's frequency rule). Angular momentum of electron is quantized: $mvr = n\frac{h}{2\pi}$, where $n = 1, 2, 3, \dots$ (principal quantum number). For Hydrogen Atom: Radii of stationary states: $r_n = n^2 a_0$, where $a_0 = 52.9 \text{ pm}$ (Bohr radius). Energy of stationary states: $E_n = -\frac{R_H}{n^2}$, where $R_H = 2.18 \times 10^{-18} \text{ J}$ (Rydberg constant). Ground state: $n=1$, lowest energy. Ionized hydrogen atom: $n=\infty$, energy $E=0$. For one-electron species (e.g., $\text{He}^+, \text{Li}^{2+}$): Energies: $E_n = -2.18 \times 10^{-18} \text{ J} \frac{Z^2}{n^2}$. Radii: $r_n = 52.9 \frac{n^2}{Z} \text{ pm}$. 4.1 Line Spectrum of Hydrogen Emission spectrum shows discrete lines. Rydberg formula for wavenumber: $\bar{\nu} = 109,677 \left(\frac{1}{n_1^2} - \frac{1}{n_2^2}\right) \text{ cm}^{-1}$. Spectral Series: Series $n_1$ $n_2$ Spectral Region Lyman 1 $2,3,\dots$ Ultraviolet Balmer 2 $3,4,\dots$ Visible Paschen 3 $4,5,\dots$ Infrared Brackett 4 $5,6,\dots$ Infrared Pfund 5 $6,7,\dots$ Infrared 4.2 Limitations of Bohr's Model Fails to explain fine details of H-atom spectrum. Unable to explain spectra of multi-electron atoms. Could not explain Zeeman effect (splitting of spectral lines in magnetic field) or Stark effect (electric field). Could not explain chemical bond formation. Ignores dual behaviour of matter and contradicts Heisenberg uncertainty principle. 5. Towards Quantum Mechanical Model of the Atom 5.1 Dual Behaviour of Matter (de Broglie Relation) de Broglie (1924) proposed that matter (like electrons) also exhibits dual behaviour (particle and wave-like properties). de Broglie Wavelength: $\lambda = \frac{h}{mv} = \frac{h}{p}$. This was experimentally confirmed by electron diffraction. 5.2 Heisenberg's Uncertainty Principle Werner Heisenberg (1927) stated that it is impossible to simultaneously determine with certainty the exact position and exact momentum (or velocity) of a microscopic particle like an electron. Mathematical expression: $\Delta x \cdot \Delta p_x \ge \frac{h}{4\pi}$ or $\Delta x \cdot m\Delta v \ge \frac{h}{4\pi}$. Implication: Rules out definite paths/trajectories for electrons. Position and velocity can only be described probabilistically. 6. Quantum Mechanical Model of Atom Based on wave-particle duality and Heisenberg uncertainty principle. Developed by Heisenberg and Schrödinger (1926). Schrödinger Equation: Describes motion of microscopic objects; $\hat{H}\Psi = E\Psi$. Solutions give allowed energy levels ($E$) and wave functions ($\Psi$). For hydrogen atom, solutions lead to quantized energy states and corresponding wave functions characterized by quantum numbers ($n, l, m_l$). Wave function ($\Psi$) itself has no physical meaning. $|\Psi|^2$ represents probability density of finding an electron at a point. Region of high $|\Psi|^2$ is where electron is most probably found. Atomic Orbitals: Wave functions for electrons in an atom. Each orbital has a definite energy. 6.1 Quantum Numbers Each electron in an atom is described by a unique set of four quantum numbers. Principal Quantum Number ($n$): Positive integer ($1, 2, 3, \dots$). Determines the size and energy of the orbital. Identifies the shell (K, L, M, N for $n=1,2,3,4$). Number of orbitals in a shell = $n^2$. Azimuthal (Orbital Angular Momentum) Quantum Number ($l$): Integer values from $0$ to $n-1$. Determines the shape of the orbital. Identifies the subshell : $l=0 \implies s$ subshell (spherical) $l=1 \implies p$ subshell (dumbbell) $l=2 \implies d$ subshell (double dumbbell) $l=3 \implies f$ subshell Number of subshells in a shell = $n$. Magnetic Orbital Quantum Number ($m_l$): Integer values from $-l$ to $+l$, including $0$. Determines the orientation of the orbital in space. Number of orbitals in a subshell = $2l+1$. For $s$ ($l=0$): $m_l=0$ (1 orbital). For $p$ ($l=1$): $m_l=-1, 0, +1$ (3 orbitals: $p_x, p_y, p_z$). For $d$ ($l=2$): $m_l=-2, -1, 0, +1, +2$ (5 orbitals: $d_{xy}, d_{yz}, d_{xz}, d_{x^2-y^2}, d_{z^2}$). Electron Spin Quantum Number ($m_s$): Values: $+\frac{1}{2}$ (spin up, $\uparrow$) or $-\frac{1}{2}$ (spin down, $\downarrow$). Represents the intrinsic spin angular momentum of the electron. 6.2 Shapes of Atomic Orbitals s-orbitals (l=0): Spherical shape. Size increases with $n$ (1s p-orbitals (l=1): Dumbbell shape, three orientations ($p_x, p_y, p_z$) along axes. Size and energy increase with $n$. Have nodal planes. d-orbitals (l=2): Five orientations. Shapes ($d_{xy}, d_{yz}, d_{xz}, d_{x^2-y^2}$) are similar, $d_{z^2}$ is unique. Total number of nodes = $(n-1)$. Angular nodes = $l$. Radial nodes = $(n-l-1)$. 6.3 Energies of Orbitals Hydrogen Atom: Energy depends only on $n$. $$1s Multi-electron Atoms: Energy depends on both $n$ and $l$ due to electron-electron repulsion and shielding effect. Order of energy within a principal quantum number: $s Shielding Effect: Inner shell electrons reduce the positive charge experienced by outer electrons. Effective Nuclear Charge ($Z_{eff}$): Net positive charge experienced by outer electrons. (n+l) Rule: Lower $(n+l)$ value, lower energy. If $(n+l)$ values are equal, orbital with lower $n$ has lower energy. 6.4 Filling of Orbitals in Atom Aufbau Principle: Orbitals are filled in order of increasing energy. Electrons occupy lowest energy orbitals first. Order: $1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, \dots$ Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. An orbital can hold a maximum of two electrons, and they must have opposite spins. Hund's Rule of Maximum Multiplicity: Electron pairing in degenerate orbitals (same subshell) does not occur until all orbitals of that subshell are singly occupied with parallel spins. Electronic Configuration: Distribution of electrons in orbitals. Notation: $nl^x$ (e.g., $1s^2$). Orbital diagram: Boxes for orbitals, arrows for electrons ($\uparrow$ for $m_s = +1/2$, $\downarrow$ for $m_s = -1/2$). Core electrons: Electrons in completely filled inner shells. Valence electrons: Electrons in the outermost shell. Stability of Completely Filled and Half-Filled Subshells: They have extra stability due to: Symmetrical distribution of electrons. High exchange energy (energy released when electrons with same spin exchange positions). Examples: $\text{Cr}$ ($[Ar]3d^54s^1$) instead of $3d^44s^2$; $\text{Cu}$ ($[Ar]3d^{10}4s^1$) instead of $3d^94s^2$.