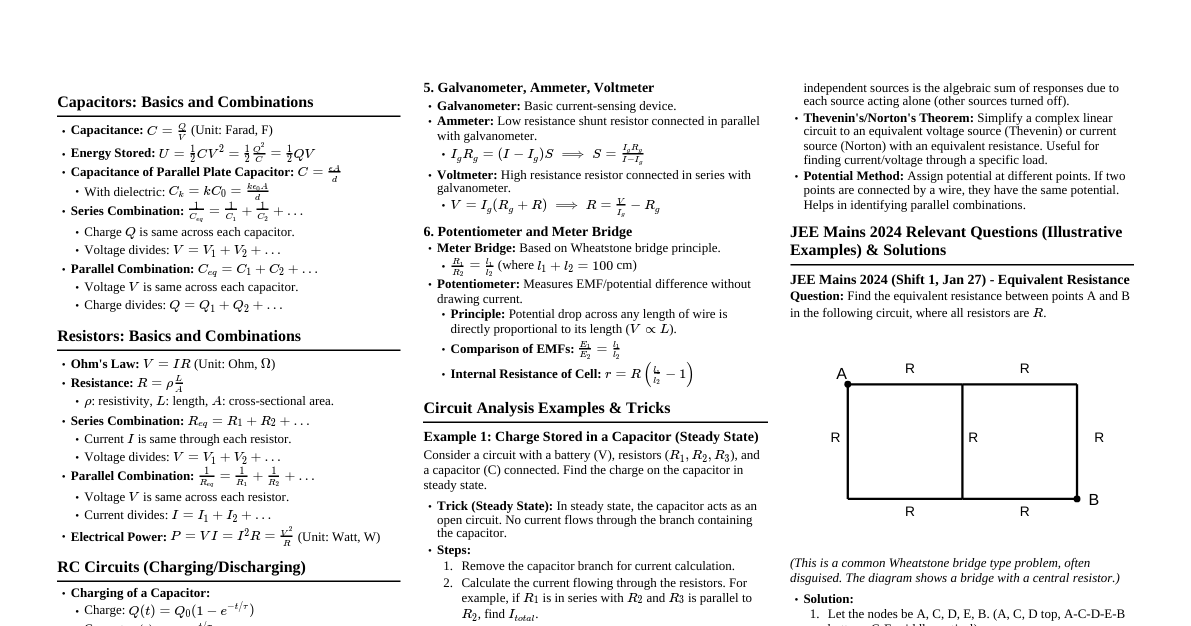
1. Fundamental Particles & Atomic Models Electron: J.J. Thomson (cathode rays), charge $e = -1.602 \times 10^{-19} C$, mass $m_e = 9.109 \times 10^{-31} kg$. Proton: E. Goldstein (anode rays), charge $+e$, mass $m_p = 1.672 \times 10^{-27} kg$. Neutron: James Chadwick, charge $0$, mass $m_n = 1.674 \times 10^{-27} kg$. Thomson's Model (Plum Pudding): Uniform sphere of positive charge with electrons embedded. Failed to explain $\alpha$-scattering. Rutherford's Model ($\alpha$-scattering): Majority space is empty. Positive charge and most mass concentrated in a tiny nucleus. Electrons revolve around the nucleus. Failed to explain stability of atom and line spectra. 2. Bohr's Model for Hydrogen Atom Postulates: Electrons revolve in fixed circular orbits (stationary states) without radiating energy. Only orbits with angular momentum $L = m_e v r = n \frac{h}{2\pi}$ ($n=1,2,3,...$) are allowed. Energy is emitted/absorbed when an electron jumps between stationary states: $\Delta E = E_2 - E_1 = h\nu$. Radius of $n^{th}$ orbit: $r_n = 0.529 \frac{n^2}{Z} \text{ Å}$ ($Z$ is atomic number). For H-atom, $r_1 = 0.529 \text{ Å}$ (Bohr radius, $a_0$). Energy of $n^{th}$ orbit: $E_n = -13.6 \frac{Z^2}{n^2} \text{ eV/atom}$. For H-atom, $E_n = -13.6 \frac{1}{n^2} \text{ eV/atom}$. Ground state ($n=1$): $E_1 = -13.6 \text{ eV}$. Ionization energy of H-atom $= 13.6 \text{ eV}$. Velocity of electron: $v_n = 2.18 \times 10^6 \frac{Z}{n} \text{ m/s}$. Limitations: Only for single electron species (H, He$^+$, Li$^{2+}$). Could not explain fine spectrum, Zeeman effect (magnetic field), Stark effect (electric field). Could not explain ability of atoms to form molecules. 3. Electromagnetic Radiation (EMR) Wave Nature: EMR travels as waves. Speed of light $c = \nu \lambda = 3 \times 10^8 \text{ m/s}$. $\nu$ (frequency), $\lambda$ (wavelength). Wavenumber $\bar{\nu} = \frac{1}{\lambda}$. Particle Nature (Planck's Quantum Theory): EMR consists of discrete packets of energy called quanta/photons. Energy of a photon $E = h\nu = \frac{hc}{\lambda}$. ($h = 6.626 \times 10^{-34} \text{ J s}$ is Planck's constant). Photoelectric Effect: Ejection of electrons from a metal surface when light of sufficient frequency (threshold frequency, $\nu_0$) shines on it. $h\nu = h\nu_0 + K.E._{max}$ $K.E._{max} = h(\nu - \nu_0) = \frac{1}{2} m_e v^2_{max}$. Work function $\phi = h\nu_0$. 4. Atomic Spectra (Hydrogen) Rydberg Formula: $\bar{\nu} = \frac{1}{\lambda} = R_H Z^2 \left( \frac{1}{n_1^2} - \frac{1}{n_2^2} \right)$ $R_H = 109677 \text{ cm}^{-1}$ (Rydberg constant). $n_1$ is lower energy level, $n_2$ is higher energy level ($n_2 > n_1$). Spectral Series for H-atom ($Z=1$): Lyman Series: $n_1=1$, $n_2=2,3,4,...$ (UV region) Balmer Series: $n_1=2$, $n_2=3,4,5,...$ (Visible region) Paschen Series: $n_1=3$, $n_2=4,5,6,...$ (IR region) Brackett Series: $n_1=4$, $n_2=5,6,7,...$ (IR region) Pfund Series: $n_1=5$, $n_2=6,7,8,...$ (IR region) Number of spectral lines: For a jump from $n_2$ to $n_1$, total lines $= \frac{(n_2-n_1)(n_2-n_1+1)}{2}$. 5. Dual Nature of Matter (de Broglie) de Broglie Wavelength: Every moving particle has wave-like properties. $\lambda = \frac{h}{mv} = \frac{h}{p}$ ($p$ is momentum). For an electron accelerated through potential $V$: $\lambda = \frac{h}{\sqrt{2m_e qV}} = \frac{12.27}{\sqrt{V}} \text{ Å}$. 6. Heisenberg's Uncertainty Principle It is impossible to simultaneously determine with absolute precision, the position and momentum of a microscopic particle. $\Delta x \cdot \Delta p \ge \frac{h}{4\pi}$ $\Delta x \cdot m \Delta v \ge \frac{h}{4\pi}$ Also, $\Delta E \cdot \Delta t \ge \frac{h}{4\pi}$. Implies that the exact path of an electron cannot be determined. 7. Quantum Mechanical Model of Atom Based on wave mechanics (Schrödinger equation). Describes electrons in terms of probabilities (orbitals). Schrödinger Equation (time-independent): $\hat{H}\Psi = E\Psi$ $\hat{H}$ is Hamiltonian operator, $\Psi$ is wave function, $E$ is energy. $\Psi^2$ (or $|\Psi|^2$) gives probability density of finding an electron in a region. 8. Quantum Numbers Describe the state of an electron in an atom. Principal Quantum Number ($n$): $n = 1, 2, 3, ...$ (K, L, M shells). Determines the main energy level and size of the orbital. Maximum electrons in a shell $= 2n^2$. Azimuthal/Angular Momentum Quantum Number ($l$): $l = 0, 1, 2, ..., (n-1)$. Determines the shape of the orbital (subshell). $l=0 \rightarrow s$ (spherical), $l=1 \rightarrow p$ (dumbbell), $l=2 \rightarrow d$ (double dumbbell), $l=3 \rightarrow f$ (complex). Number of subshells in a shell $= n$. Maximum electrons in a subshell $= 2(2l+1)$. Magnetic Quantum Number ($m_l$): $m_l = -l, ..., 0, ..., +l$. Determines the orientation of the orbital in space. Number of orbitals in a subshell $= (2l+1)$. Number of orbitals in a shell $= n^2$. Spin Quantum Number ($m_s$): $m_s = +\frac{1}{2}$ (spin up) or $-\frac{1}{2}$ (spin down). Describes the intrinsic angular momentum (spin) of the electron. 9. Rules for Filling Orbitals Aufbau Principle: Electrons fill orbitals in order of increasing energy. Order: $1s $(n+l)$ rule: Lower $(n+l)$ value has lower energy. If $(n+l)$ is same, lower $n$ has lower energy. Pauli's Exclusion Principle: No two electrons in an atom can have all four quantum numbers identical. An orbital can hold a maximum of two electrons, and they must have opposite spins. Hund's Rule of Maximum Multiplicity: Pairing of electrons in a degenerate set of orbitals ($p, d, f$) does not occur until each orbital in the subshell is singly occupied with parallel spins. 10. Electronic Configuration The distribution of electrons into orbitals. Examples: H ($Z=1$): $1s^1$ He ($Z=2$): $1s^2$ N ($Z=7$): $1s^2 2s^2 2p^3$ O ($Z=8$): $1s^2 2s^2 2p^4$ Cr ($Z=24$): $[Ar] 3d^5 4s^1$ (exception due to half-filled stability) Cu ($Z=29$): $[Ar] 3d^{10} 4s^1$ (exception due to fully-filled stability) 11. Radial & Angular Nodes Radial Nodes (Spherical Nodes): Regions where the probability of finding an electron is zero. Number of radial nodes $= n - l - 1$. Angular Nodes (Nodal Planes): Planes passing through the nucleus where the probability of finding an electron is zero. Number of angular nodes $= l$. Total Nodes: Number of total nodes $= n - 1$.