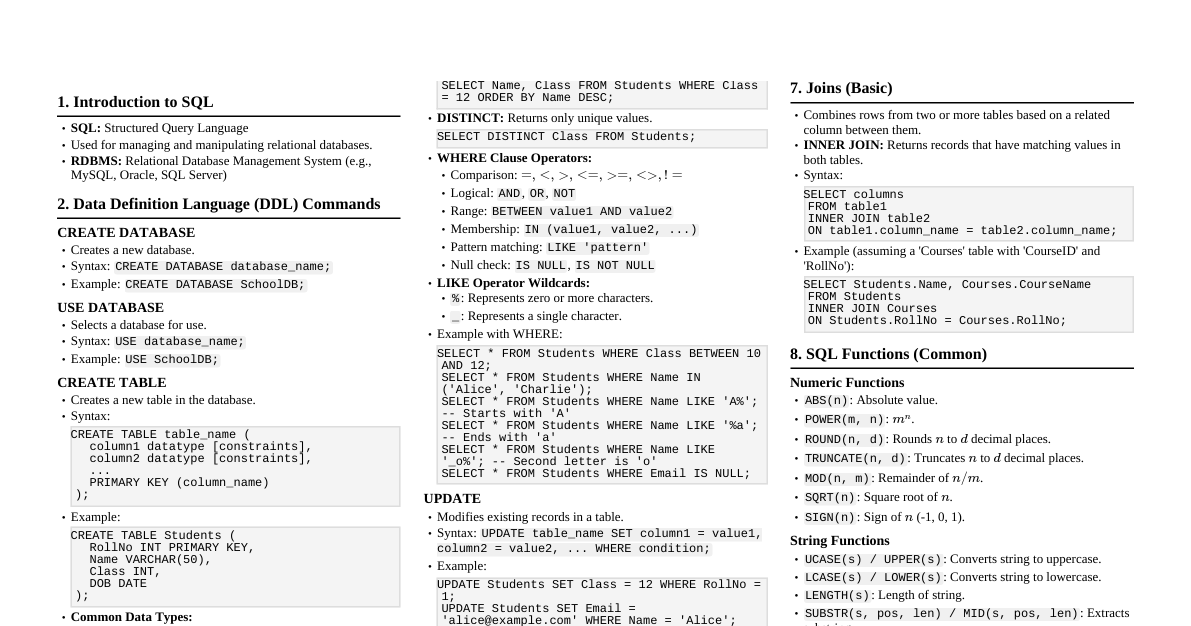
### Introduction to Hydrogen - **Symbol:** H - **Atomic Number:** 1 - **Electronic Configuration:** 1s¹ (one electron in the first shell) - **Position in Periodic Table:** First element, usually placed above Group 1 (alkali metals) but behaves differently. - **Nature:** Lightest and most abundant element in the universe. ### Occurrence of Hydrogen - **Free State:** Very rare on Earth due to its lightness and reactivity. Found in small amounts in volcanic gases. - **Combined State:** - **Water:** H₂O (most common compound) - **Acids:** HCl, H₂SO₄, HNO₃ - **Alkalies:** NaOH, KOH - **Organic Compounds:** Hydrocarbons (methane, ethane), carbohydrates (glucose), proteins, fats. - **Sun and Stars:** Major component, undergoing nuclear fusion. ### Laboratory Preparation 1. **From Acids:** - **Reaction:** Metal + Dilute Acid → Salt + Hydrogen gas - **Examples:** - Zn + H₂SO₄ (dilute) → ZnSO₄ + H₂↑ - Mg + 2HCl (dilute) → MgCl₂ + H₂↑ - **Conditions:** Zinc (granulated) with dilute sulphuric acid is preferred. Copper does not react with dilute acids. - **Collection:** By downward displacement of water (as it's insoluble in water) or upward displacement of air (as it's lighter than air). 2. **From Alkalies:** - **Reaction:** Amphoteric Metal + Alkali → Salt + Hydrogen gas - **Example:** 2Al + 2NaOH + 2H₂O → 2NaAlO₂ (Sodium aluminate) + 3H₂↑ ### Industrial Preparation 1. **Electrolysis of Water:** - **Reaction:** 2H₂O (liquid) $\xrightarrow{\text{electricity}}$ 2H₂ (gas) + O₂ (gas) - **Conditions:** Acidified or alkalized water for better conductivity. - **Products:** Hydrogen at cathode, Oxygen at anode. 2. **Bosch Process (from Steam and Hydrocarbons):** - **Step 1: Water Gas Production:** - C (coke) + H₂O (steam) $\xrightarrow{\text{1000°C}}$ CO + H₂ (Water gas) - **Step 2: Conversion of CO to CO₂:** - CO + H₂ + H₂O (excess steam) $\xrightarrow{\text{450°C, Fe₂O₃ catalyst}}$ CO₂ + 2H₂ - **Step 3: Removal of CO₂:** - Pass through water under pressure (CO₂ dissolves). - Pass through KOH solution (CO₂ reacts to form K₂CO₃). ### Physical Properties - **State:** Colourless, odourless, tasteless gas. - **Density:** Lightest known gas (lighter than air). - **Solubility:** Sparingly soluble in water. - **Combustibility:** Highly combustible, burns with a pale blue flame. - **Non-supporter of Combustion:** Does not support burning. ### Chemical Properties 1. **Combustion:** - 2H₂ + O₂ → 2H₂O (Highly exothermic reaction) - Burns with a pop sound when mixed with air/oxygen. 2. **Reaction with Non-metals:** - **Halogens:** H₂ + Cl₂ $\xrightarrow{\text{sunlight}}$ 2HCl (Hydrogen chloride) - **Nitrogen:** N₂ + 3H₂ $\xrightarrow{\text{Fe catalyst, high temp/pressure}}$ 2NH₃ (Ammonia - Haber Process) 3. **Reducing Agent:** - Removes oxygen from metal oxides (reduces them). - CuO + H₂ → Cu + H₂O - Fe₂O₃ + 3H₂ → 2Fe + 3H₂O ### Uses of Hydrogen - **Haber Process:** For the manufacture of ammonia (NH₃), which is used in fertilizers. - **Hydrogenation of Oils:** Converts vegetable oils into solid fats (margarine, vanaspati ghee) using Ni catalyst. - **Fuel:** As rocket fuel (liquid hydrogen) and in fuel cells to generate electricity. - **Metallurgy:** As a reducing agent in the extraction of metals. - **Welding:** In oxy-hydrogen flame for cutting and welding metals (produces very high temperature). - **Methanol Production:** CO + 2H₂ → CH₃OH (Methanol) - **Hydrochloric Acid Production:** H₂ + Cl₂ → 2HCl ### Safety Precautions (Lab) - Hydrogen is highly flammable. - Always check for leaks. - Do not bring a naked flame near hydrogen gas. - Ensure proper ventilation.