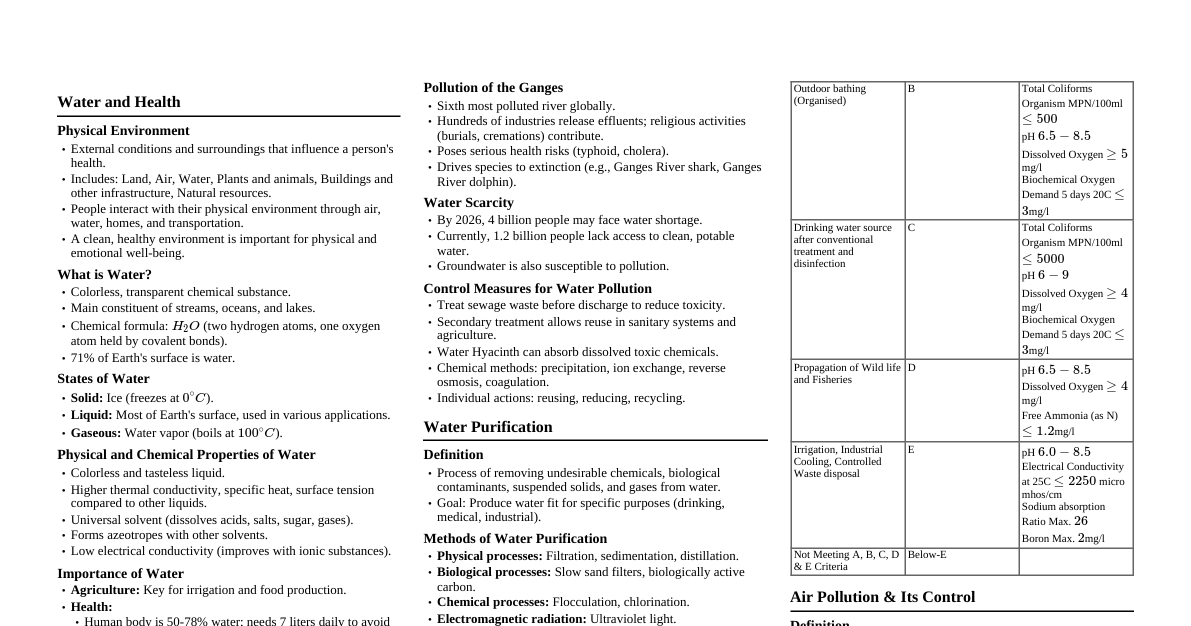
### Introduction to Environmental Science Environmental science is the study of the interactions between the physical, chemical, and biological components of the environment, including their effects on organisms and human impact. #### Environment Definition The environment encompasses all substances and forces external to an organism that affect its existence. For humans, this includes air, land, water, flora, and fauna, which regulate life. #### Classification of Environment The environment is broadly classified into four major segments: - **Atmosphere:** The gaseous layer surrounding Earth. - **Hydrosphere:** All water on Earth's surface, underground, and in the air. - **Lithosphere:** Earth's solid outer layer, including the crust and upper mantle (rocks, soils). - **Biosphere:** The sum of all ecosystems; the zone of life on Earth. ### Pollution: Basics and Types #### Definition of Pollution Pollution is the introduction of waste or unwanted matter into the environment, causing damage or deterioration to living systems and the environment. #### Definition of Pollutant A pollutant is a substance present in concentrations greater than its natural level, having a detrimental effect on the environment or being hazardous to human health, safety, or quality of life. It may harm organisms or exceed environmental quality standards. The term is often used synonymously with contaminant. #### Contaminant vs. Pollutant - A **contaminant** is a substance present in concentrations above its natural level due to human activities, causing a deviation from the normal composition of an environment. - A substance is not a pollutant unless it has some detrimental effect. - All pollutants are contaminants, but not all contaminants are pollutants. - The medium affected by a pollutant or contaminant (e.g., soil, fish) is called a **receptor**. - The chemical medium or species that retains or interacts with a pollutant/contaminant is known as a **sink**. #### Main Types of Environmental Pollution 1. **Air Pollution** 2. **Water Pollution** 3. **Soil/Land Pollution** #### Other Kinds of Pollution - **Noise Pollution:** Harmful or annoying sound levels from traffic, industry, construction, and loudspeakers, leading to stress, sleep disturbance, and hearing loss. - **Light Pollution:** Excessive or misdirected artificial lighting that disrupts ecosystems and human circadian rhythms. - **Thermal Pollution:** Unwanted heating of water bodies, often from the discharge of hot effluents from power plants or industries, reducing dissolved oxygen and stressing aquatic life. - **Radioactive Pollution:** Presence of radioactive substances in air, water, or soil from nuclear accidents, improper waste disposal, or certain industrial/medical uses, leading to cancer and genetic effects. - **Plastic Pollution:** Accumulation of plastic waste in land and oceans, causing entanglement, ingestion by wildlife, and microplastic formation. - **Visual Pollution:** Unpleasant changes in landscapes from billboards, overhead wires, unmanaged waste, and cluttered urban infrastructure. ### Atmosphere #### Role of the Atmosphere - **Protective Blanket:** Nurtures life on Earth and protects it from the hostile environment of outer space. - **Source of Gases:** Provides carbon dioxide for plant photosynthesis and oxygen for respiration. - **Nitrogen Source:** Supplies nitrogen for nitrogen-fixing bacteria and ammonia-manufacturing plants, essential for life molecules. #### Importance of the Atmosphere - **Hydrologic Cycle:** Transports water from oceans to land, acting as a condenser in the solar-powered hydrologic cycle. - **Pollutant Sink:** Historically served as a dumping ground for pollutant materials like sulfur dioxide and refrigerants (Freons). This practice causes damage to vegetation and materials, shortens human life, and alters atmospheric characteristics. #### Earth's Atmosphere: A Thin Veneer - Earth's radius is approximately 6400 km (3840 miles). - Nearly all the atmosphere is contained within the first 100 km from the surface. - The habitable atmosphere is only the first 5 km. - Thus, the habitable atmosphere is roughly 5/6400 km or 1/1280th of the distance to Earth's center, much thinner than an orange peel. #### Vertical Structure of the Atmosphere - **Troposphere:** The lowest layer, where weather occurs. Temperature generally decreases with altitude. - **Stratosphere:** Above the troposphere, temperature rises with increasing altitude due to the ozone layer absorbing solar UV radiation. - **Mesosphere:** Above the stratosphere, temperatures decrease with altitude. Few gas molecules here absorb solar radiation, so the heat source is the stratosphere below. - **Thermosphere:** The outermost layer, temperature increases rapidly due to absorption of high-energy solar radiation by nitrogen and oxygen atoms. #### Bulk Composition of Unpolluted Air Unpolluted dry air is primarily composed of: - **Nitrogen (N₂):** ~78.084% by volume - **Oxygen (O₂):** ~20.946% by volume - **Argon (Ar):** ~0.934% by volume - **Carbon Dioxide (CO₂):** ~0.0397% by volume (variable) - **Neon (Ne):** ~0.001818% by volume - **Helium (He):** ~0.000524% by volume - **Methane (CH₄):** ~0.000179% by volume - **Water vapor (H₂O):** Highly variable, typically 0.001%-5% by volume. Not included in dry atmosphere composition. ### Air Pollutants #### Primary Pollutants Pollutants emitted directly to the atmosphere in urban environments. - **Example:** Smoke. #### Secondary Pollutants Formed when primary pollutants undergo reactions in the atmosphere. - Many primary pollutants can react to produce secondary pollutants. #### Categories of Air Pollutants ##### Primary Particles - Pollen - Dust - Fly ash - Polycyclic aromatic hydrocarbons ##### Secondary Particles (formed from gas reactions) - Smog particles - Sulfuric acid droplets - Salts such as (NH₄)₂SO₄ ##### Inorganic Gases - Ozone (O₃) - Sulfur Dioxide (SO₂) - Nitrogen Oxide (NO) - Nitrogen Dioxide (NO₂) - Carbon Monoxide (CO) - Hydrogen Sulfide (H₂S) - Hydrogen Chloride (HCl) - Ammonia (NH₃) ##### Organics - Hydrocarbons (including those forming photochemical smog) - Odorous organic sulfur compounds - Organohalides - Amines and other organonitrogen compounds - Organo-oxygen compounds (including aldehydes and ketones) ##### Photochemical Smog - Smog particles - Ozone (O₃) - Organic oxidants (PAN - Peroxyacetyl Nitrate) - Aldehydes ### Sources of Air Pollutants #### Natural Sources - **Geochemical sources:** Wind-blown dusts and sea sprays. - **Biological sources:** Forest fires, a significant source of carbon (soot particles). #### Anthropogenic Sources - **Point Source:** Emissions from a single, identifiable source (e.g., a factory smokestack). - **Area Source:** Emissions from diffuse sources over a geographical area (e.g., residential heating, small businesses). - **Mobile Source:** Emissions from moving vehicles (e.g., cars, trucks, airplanes). ### Air Quality Standards and Index #### National Ambient Air Quality Standards (NAAQS) by CPCBs These standards define permissible concentrations of various pollutants in ambient air, differentiating between industrial/residential/rural areas and ecologically sensitive areas. | Pollutant | Time Weighted Average | Industrial, Residential, Rural Areas | Ecologically Sensitive Area | |:----------|:----------------------|:-----------------------------------|:----------------------------| | SO₂ | Annual* | 50 µg/m³ | 20 µg/m³ | | | 24 hours** | 80 µg/m³ | 80 µg/m³ | | NO₂ | Annual* | 40 µg/m³ | 30 µg/m³ | | | 24 hours** | 80 µg/m³ | 80 µg/m³ | | PM₁₀ | Annual* | 60 µg/m³ | 60 µg/m³ | | | 24 hours** | 100 µg/m³ | 100 µg/m³ | | PM₂.₅ | Annual* | 40 µg/m³ | 40 µg/m³ | | | 24 hours** | 60 µg/m³ | 60 µg/m³ | | O₃ | 8 hours* | 100 µg/m³ | 100 µg/m³ | | | 1 hour** | 180 µg/m³ | 180 µg/m³ | | Pb | Annual* | 0.5 µg/m³ | 0.5 µg/m³ | | | 24 hours** | 1.0 µg/m³ | 1.0 µg/m³ | | CO | 8 hours* | 02 mg/m³ | 02 mg/m³ | | | 1 hour** | 04 mg/m³ | 04 mg/m³ | | NH₃ | Annual* | 100 µg/m³ | 100 µg/m³ | | | 24 hours** | 400 µg/m³ | 400 µg/m³ | | C₆H₆ | Annual* | 5 µg/m³ | 5 µg/m³ | | BaP | Annual* | 1 ng/m³ | 1 ng/m³ | | As | Annual* | 6 ng/m³ | 60 ng/m³ | | Ni | Annual* | 20 ng/m³ | 20 ng/m³ | *Notes:* - \* Annual arithmetic mean of minimum 104 measurements in a year at a particular site, taken twice a week 24 hourly at uniform intervals. - \*\* 24 hourly or 8 hourly or 1 hourly monitored values, as applicable, shall be complied with 98% of the time. They may exceed the limits but not on two consecutive days of monitoring. #### Air Quality Index (AQI) The AQI is a standardized numerical scale used to communicate how polluted the air currently is or is forecasted to be. It makes complex air pollution data accessible to the general public. AQI is calculated as the maximum of the sub-indices of individual pollutants. **AQI Categories:** | AQI Range | Category | |:----------|:--------------| | 0-50 | Good | | 51-100 | Satisfactory | | 101-200 | Moderate | | 201-300 | Poor | | 301-400 | Very Poor | | 401-500 | Severe | #### Natural Factors That Reduce Air Pollution - Particles heavier than air - Rain and snow - Winds - Chemical reactions #### Natural Factors That Increase Air Pollution - Urban buildings - Hills and mountains - High temperatures - VOC emissions from certain trees and plants - Temperature inversions ### Air Pollution Impact #### Effects of Air Pollution * Acid deposition * Ozone layer depletion * Global warming and the greenhouse effect #### Acid Rain ##### What is Acid Rain? * Unpolluted rainwater is naturally slightly acidic (pH 5.7) due to dissolved carbon dioxide forming carbonic acid ($$\text{CO}_2 + \text{H}_2\text{O} \rightarrow \text{H}_2\text{CO}_3$$). * Rainwater with pH as low as 2.5 has been recorded in some parts of the world, commonly known as acid rain. * "Acid rain" is a broad term; **acid deposition** is more precise, including wet and dry forms. * **Wet deposition:** Acidic rain, fog, and snow. * **Dry deposition:** Acidic gases and particles. ##### Causes of Acid Rain * **Primary Causes:** Sulphur dioxide ($\text{SO}_2$) and nitrogen oxides ($\text{NO}_x$). * **Formation:** Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds (e.g., sulfuric acid, nitric acid). * **Rate Enhancement:** Sunlight increases the rate of most of these reactions. ##### Acid Deposition Process Flowchart 1. **Emissions:** Sulfur dioxide ($\text{SO}_2$) and nitrogen oxides ($\text{NO}_x$) are emitted into the atmosphere. 2. **Transformation:** These gases are transformed into sulfuric acid ($\text{H}_2\text{SO}_4$) and nitric acid ($\text{HNO}_3$). 3. **Windborne Transport:** Wind carries ammonia gas and soil particles, which partially neutralize acids and form dry sulfate and nitrate salts. 4. **Dry Deposition:** Sulfur dioxide gas and particles of sulfate and nitrate salts deposit directly onto surfaces. 5. **Wet Deposition:** Droplets of $\text{H}_2\text{SO}_4$ and $\text{HNO}_3$ dissolve in rain and snow, falling as acid rain. ##### Impacts of Air Pollution on Trees and Water **Flowchart: Impacts on Terrestrial and Aquatic Ecosystems** * **Emissions** ($\text{SO}_2$, $\text{NO}_x$, $\text{H}_2\text{O}_2$, $\text{O}_3$, PANs, Others) lead to: * **Acid deposition** (on leaves and bark) * **Direct damage to leaves and bark** * **Reduced photosynthesis and growth** * **Increased susceptibility** to drought, extreme cold, insects, mosses, and disease organisms * **Soil acidification** (degrading soil quality, making it infertile) * Leaching of soil nutrients * Release of toxic metal ions * **Root damage** * **Reduced nutrient and water uptake** * **Tree death** * **Groundwater** and **Lake** (Lakes in shallow soil low in limestone become acidic; Lakes in deep soil high in limestone are buffered.) ##### Reducing Acid Deposition **Prevention:** * Reduce coal use * Burn low-sulfur coal * Increase natural gas use * Increase use of renewable energy resources * Remove $\text{SO}_2$ particulates and $\text{NO}_x$ from smokestack gases * Remove $\text{NO}_x$ from motor vehicular exhaust * Tax emissions of $\text{SO}_2$ * Reduce air pollution by improving energy efficiency **Cleanup:** * Add lime to neutralize acidified lakes * Add phosphate fertilizer to neutralize acidified lakes #### Photochemical Smog ##### What is Photochemical Smog? * Aerosols play a role. * Photochemical smog is a mixture of pollutants, including particulates, nitrogen oxides ($\text{NO}_x$), ozones, aldehydes, peroxyacetyl nitrate (PAN), and unreacted hydrocarbons. Sunlight must be present for its formation. * Indicators: A brownish haze and painful eyes. Nitrogen dioxide is responsible for the brownish color. ##### Causes of Photochemical Smog * **Initiation:** Reactions are initiated by sunlight. * **Components:** Involve hydrocarbons and nitrogen oxides emitted from automobiles. * **Favorable Conditions:** The combination of sunlight, catalysis by particulates, and abundant pollutants in modern cities provides favorable conditions for smog formation. ##### Photochemical Reactions * Oxygen ($\text{O}_2$) does not react very fast in the atmosphere. * Oxygen can be photochemically converted to small amounts of ozone ($\text{O}_3$). $\text{O}_3$ is a very reactive gas and can initiate other processes. * In the stratosphere, $\text{O}_3$ is beneficial as it filters UV light. At Earth's surface, its reactivity makes it harmful to living things. ##### Example of Photochemical Smog Reactions * $$\text{O} + \text{O}_2 \rightarrow \text{O}_3$$ (Ozone) * $$\text{O} + \text{hydrocarbons} \rightarrow \text{aldehydes}$$ * $$\text{O}_3 + \text{hydrocarbons} \rightarrow \text{aldehydes}$$ * $$\text{NO}_2 + \text{O}_2 + \text{hydrocarbons} \rightarrow \text{peroxyacetyl nitrate (PAN)}$$ (PAN: $\text{CH}_3\text{C(O)OONO}_2$) ##### Environmental Problems Caused by Photochemical Smog * **Health:** Headaches, eye, nose, and throat irritations, impaired lung function, coughing, and wheezing. * **Materials:** Causes rubbers and fabrics to deteriorate. * **Agriculture:** Damages plants, leading to crop loss. #### Greenhouse Effect and Global Warming ##### Greenhouse Effect * **Definition:** A natural process that allows Earth to retain some of the heat from the sun. * **Mechanism:** Gases in the atmosphere (water vapor, carbon dioxide, nitrous oxide, and methane) trap energy from the sun. Without these gases, heat would escape back into space, and Earth's average temperature would be about -18 °C. With the atmosphere, it's about 15 °C. * **Greenhouse Gases:** These gases are referred to as greenhouse gases because of their warming effect. ##### Greenhouse Effect vs. Global Warming * The "greenhouse effect" and "global warming" are not the same thing. * **Global warming:** Refers to a rise in the temperature of the Earth's surface. * An increase in the concentration of greenhouse gases leads to an increase in the magnitude of the greenhouse effect (called **enhanced greenhouse effect**). This results in global warming. ##### Carbon Dioxide (CO₂) * **Primary Greenhouse Gas:** Carbon dioxide is considered a primary greenhouse gas. * **Heat-Trapping Ability (Relative to CO₂):** * Methane ($\text{CH}_4$): 23 times * Nitrous oxide ($\text{N}_2\text{O}$): 296 times * HFC-23: 12,000 times * **Note:** While some gases have higher heat-trapping potential per molecule, their atmospheric concentration is much lower than $\text{CO}_2$, making $\text{CO}_2$ the primary driver of global warming. ##### Global Warming Potential (GWP) * **Definition:** The ratio of global warming from one unit mass of a greenhouse gas to that of one unit mass of carbon dioxide over a period of time. It measures the potential for global warming per unit mass relative to carbon dioxide. ##### Absorption Spectra of Atmospheric Gases * Different atmospheric gases absorb infrared (IR) radiation at different wavelengths. * Gases like $\text{CH}_4$, $\text{N}_2\text{O}$, $\text{O}_2 \& \text{O}_3$, $\text{CO}_2$, and $\text{H}_2\text{O}$ have distinct IR absorption peaks. * More and stronger IR absorption peaks indicate a higher global warming potential. * **IR Windows:** Regions in the spectrum where the atmosphere is transparent to IR radiation. ##### Atmospheric Carbon Dioxide Levels * Historical data from Vostok Ice Core and Law Dome Ice Core show natural fluctuations over hundreds of thousands of years. * Mauna Loa Instrumental Measurements show a significant, rapid increase in $\text{CO}_2$ levels, especially since the Industrial Revolution. * Current levels (e.g., 428 ppm in Jan 2026) are significantly higher than pre-industrial levels (~278 ppm). * A doubling of pre-industrial $\text{CO}_2$ levels is projected to significantly increase Earth's temperature (e.g., ~2°C). ##### Climate Change vs. Variability * **Climate variability:** Natural fluctuations in climate. Even in a stable climate, there are variations (wet/dry years, warm/cold years). * **Climate change:** Refers to a long-term shift in global or regional climate patterns, often attributed to human activities. * **Challenge:** Scientists aim to determine if changes in precipitation, temperature, frequency of storms, sea level, etc., are due to climate variability or climate change. ##### Atmospheric Feedbacks **Flowchart: Atmospheric Feedbacks** **Positive Feedback Loop:** 1. **Increased $\text{CO}_2$** 2. Leads to **Higher temperature** 3. Leads to **More water vapor** (a greenhouse gas) 4. More water vapor leads to even **Higher temperature** 5. This drives **More water vapor** and **More absorbed infrared radiation** **Negative Feedback Loop:** 1. **Increased $\text{CO}_2$** 2. Leads to **Higher temperature** 3. Leads to **More water vapor** 4. Leads to **Increased cloud cover** (increases albedo) 5. Leads to **More reflected solar radiation** (decreases absorbed solar radiation) 6. Leads to **Lower temperature** 7. Leads to **Less water vapor** * **Albedo:** The reflectivity of a surface. About 30% of Earth's albedo is natural (e.g., deserts, ice). Albedo is wavelength-dependent. ##### Human Activity and Greenhouse Effect * **Mechanism:** Human activities lead to increased greenhouse gas concentrations, enhancing the natural greenhouse effect. * **Sources:** * **Deforestation:** Reduces $\text{CO}_2$ absorption. * **CFCs:** Potent greenhouse gases and ozone depleters. * **Oil and petrol engines:** Release $\text{CO}_2$, $\text{NO}_x$, hydrocarbons. * **Fossil fuels:** Burning releases large amounts of $\text{CO}_2$. * **Radiation Balance:** Humans are altering the balance of absorbed and reflected radiation, leading to increased radiation absorbed by greenhouse gases and the Earth. ##### CO₂ Sources * **Natural:** Forest fires, volcanic eruptions, cellular respiration. * **Anthropogenic:** Increase in fossil fuel usage and deforestation shifts the balance of $\text{CO}_2$ in the atmosphere. #### Ozone Depletion ##### Ozone Depletion Process Flowchart 1. **Factories and homes spew out CFCs.** 2. **Sunlight breaks down CFCs in the stratosphere**, releasing chlorine atoms. 3. **Chlorine atoms attack ozone molecules**, breaking them down. 4. **Scientists send up balloons to see where all the ozone has gone.** ##### Destruction of Ozone Layer * **Mechanism:** Chlorine atoms from CFCs attack ozone ($\text{O}_3$), taking away an oxygen atom and forming chlorine monoxide (ClO). * $$\text{O}_3 + \text{Cl} \rightarrow \text{O}_2 + \text{ClO}$$ * **Regeneration of Chlorine:** Chlorine monoxide then combines with another oxygen atom to form a new oxygen molecule and regenerate the chlorine atom. * $$\text{ClO} + \text{O} \rightarrow \text{Cl} + \text{O}_2$$ * **Chain Reaction:** A single chlorine atom can destroy up to 100,000 ozone molecules. ##### What are CFCs? * **Chlorofluorocarbons (CFCs):** Composed of chlorine, fluorine, and carbon. * **Atmospheric Stability:** CFCs remain stable in the troposphere due to unreactivity. * **Stratospheric Transport:** Over 11-20 years, they are lifted into the stratosphere by convection and drift. * **Breakdown:** In the stratosphere, UV rays break them down, releasing highly reactive chlorine atoms that initiate a chain reaction to break apart ozone. * **Usage:** * Refrigerants * Blowing agents * For making flexible foam * Cleaning agents * Propellants ##### Consequences of Less Ozone * **Increased UV-B Radiation:** Because CFCs have a long lifespan and are stable, they continuously attack the ozone layer, leading to more UV-B radiation reaching Earth. * **Health Impacts:** Sunburn, eye diseases (cataracts), skin cancer. * **Immune System:** Reduces the human immune system, leading to other diseases. * **Ecosystems:** Reduces photosynthesis (crops affected), kills plankton (impacting marine food webs and fish populations). ### Motor Vehicle Emissions and Control #### Primary Types of Air Pollutants from Vehicles * **Carbon Monoxide (CO)** * **Volatile Organic Compounds (VOCs):** Includes BTEX (Benzene, Toluene, Ethylbenzene, Xylenes). * **Oxides of Nitrogen ($\text{NO}_x$)** * **Sulfur Dioxide ($\text{SO}_2$)** * **Particulate Matter ($\text{PM}_{10}$)** * **Lead (Pb)** * **Note:** $\text{CO}_2$ is not considered a pollutant in the same way as the others; it's a greenhouse gas. A contaminant that causes no health effect is not a pollutant. #### Extent of Air Pollution Today (Mobile vs. Stationary Sources) * Mobile sources contribute significantly to various pollutants. For example, in 1990, mobile sources accounted for 54 million metric tons, or 43% of total emissions. #### Fuel Consumption and Miles Traveled * There has been a continuous increase in miles traveled and fuel used over decades, indicating growing emissions. #### The Combustion Process (Actual) **Flowchart: Combustion Process in a Vehicle** * **Today's Air** and **Real Fuel** enter the engine. * **Combustion** occurs. * **Exhaust** is produced, containing: * Nitrogen * Water (steam) * Carbon Dioxide * Pollutants * **Pollutants** released include: * Unburned Hydrocarbons * Carbon Monoxide * Oxides of Nitrogen * Other elements or compounds (e.g., PAN) #### The Motor Vehicle as a Source of Air Pollution **Flowchart: Sources of Vehicle Emissions** * **Refueling Losses:** Emissions during fuel tank filling (petrol is volatile, so there's some loss due to evaporation). * **Evaporative Emissions:** Fuel evaporation from the fuel system when the vehicle is not running. * **Exhaust Emissions:** Gases and particles released directly from the tailpipe. * **Crankcase Losses:** Blow-by gases escaping from the engine's crankcase. #### The Combustion Process (Theoretical - Complete Combustion) * **Gasoline/Petrol:** (e.g., $\text{C}_7\text{H}_{13}$) * **Air:** Oxygen ($\text{O}_2$) and Nitrogen ($\text{N}_2$) * **Reaction:** $$\text{C}_7\text{H}_{13} + 10 \text{O}_2 + 39 \text{N}_2 \Rightarrow \text{Energy!!}$$ * **Products:** $$7 \text{CO}_2 + 6.5 \text{H}_2\text{O} + 39 \text{N}_2$$ * Carbon Dioxide * Water (Steam) * Nitrogen #### How Emissions are Formed * **In the engine:** * Incomplete combustion * "Wall quench" (combustion near cool cylinder walls) * High pressure and temperature * "Blowby" (gases escaping past piston rings) * **Due to evaporation of fuel:** * "Breathing" (vapor release from fuel tank due to temperature changes) * Hot engine and fuel (evaporation from hot surfaces) * Displacement of vapors (during refueling) #### Air-Fuel Ratio (AFR) * **Definition:** The mass ratio of air to fuel present during combustion. * **Stoichiometric Mixture:** The ratio where exactly enough air is provided to completely burn all fuel. For pure octane, this is approximately 14.7:1. * **Lambda ($\lambda$):** An alternative way to represent AFR. $\lambda = 1$ for stoichiometric. * **Importance:** Crucial for anti-pollution and performance tuning. * **Maximum Power:** Reached at AFRs ranging from 12.5 - 13.3:1 (or $\lambda$ of 0.850 - 0.901). **Graph: Effect of Air-Fuel Ratio on Emissions and Fuel Economy** * **X-axis:** Rich (too much fuel) $\leftarrow$ Stoichiometric (14.7 to 1) $\rightarrow$ Lean (too much air) * **Y-axis:** Higher $\uparrow$ Lower * **Curves:** - **Fuel Economy:** Peaks slightly lean of stoichiometric. - **NOx:** Peaks lean of stoichiometric (high temperature). - **HC:** High at rich mixtures, drops near stoichiometric, high at lean mixtures (misfires). - **CO:** High at rich mixtures, drops sharply to low levels at stoichiometric and lean. * **Note:** Petrol engines operate near stoichiometric (14.7:1); diesel engines operate at high air/fuel ratios (e.g., 30:1). #### Internal Combustion Engine (ICE) * **Mechanism:** Fuel combustion occurs with an oxidizer (air) in a combustion chamber. The expansion of high-temperature and -pressure gases produces mechanical energy by applying force to components like pistons, turbine blades, or nozzles. #### External Combustion Engine (ECE) * **Mechanism:** A working fluid is heated by an external source (e.g., coal combustion) through a heat exchanger. The fluid expands, drives a mechanism (e.g., piston), and then is cooled, compressed, and reused (closed cycle) or dumped (open cycle). * **Example:** Steam engine (e.g., earlier nuclear power plants used this concept, PHWR). #### Vehicular Emission Control Measures 1. Exhaust gas recirculation (EGR) 2. Air injection 3. Catalytic converter ##### 1. Exhaust Gas Recirculation (EGR) * **Purpose:** A nitrogen oxide ($\text{NO}_x$) emissions reduction technique used in petrol/gasoline and diesel engines. * **Mechanism:** Recirculates a portion of an engine's exhaust gas back to the engine cylinders. * **Effect:** Dilutes oxygen ($\text{O}_2$) in the incoming air stream and provides inert gases to absorb combustion heat, reducing peak in-cylinder temperatures. * **$\text{NO}_x$ Production:** Depends on temperature; lower temperature leads to less $\text{NO}_x$. ##### 2. Air Injection * **Purpose:** One of the earliest emission control systems. * **Mechanism:** Injects air into the engine's exhaust ports to provide oxygen. * **Effect:** Allows unburned and partially burned hydrocarbons in the exhaust to finish burning. ##### 3. Catalytic Converters * **Mechanism:** A device in the exhaust pipe that converts harmful pollutants (hydrocarbons, carbon monoxide, $\text{NO}_x$) into less harmful gases using platinum (Pt), palladium (Pd), and rhodium (Rh) as catalysts. ###### Types of Catalytic Converters * **Two-way catalytic converter:** * **Tasks:** 1. Oxidation of carbon monoxide to carbon dioxide: $$2\text{CO} + \text{O}_2 \Rightarrow 2\text{CO}_2$$ 2. Oxidation of unburnt hydrocarbons to carbon dioxide and water: $$\text{C}_x\text{H}_{2x+2} + [(3x+1)/2]\text{O}_2 \Rightarrow x\text{CO}_2 + (x+1)\text{H}_2\text{O}$$ * **Usage:** Widely used on diesel engines. * **Limitation:** Unable to control nitrous oxide ($\text{NO}_x$) emissions, which is a problem at low temperatures. Superseded by three-way converters. * **Three-way catalytic converter:** * **Tasks:** (Most efficient when engine runs slightly above stoichiometric point) 1. **Oxidation** of carbon monoxide to carbon dioxide: $$2\text{CO} + \text{O}_2 \Rightarrow 2\text{CO}_2$$ 2. **Oxidation** of unburnt hydrocarbons to carbon dioxide and water: $$\text{C}_x\text{H}_{2x+2} + [(3x+1)/2]\text{O}_2 \Rightarrow x\text{CO}_2 + (x+1)\text{H}_2\text{O}$$ 3. **Reduction** of nitrogen oxides to nitrogen and oxygen: $$2\text{NO}_x \Rightarrow x\text{O}_2 + \text{N}_2$$ * **Usage:** Common in North America and many other countries. #### Emission Norms ##### Bharat Stage Emission Standards (BSES) * **Purpose:** Emission standards instituted by the Government of India to regulate air pollutant output from internal combustion and spark-ignition engines in motor vehicles. * **Authority:** Set by the Central Pollution Control Board under the Ministry of Environment & Forests and Climate Change. * **Figures:** All figures are in g/km. **Table: Petrol Emission Norms (g/km)** | Norm | CO | HC | NOx | HC+NOx | PM | |:---------|:-------|:-------|:-------|:-------|:-------| | BS-III | 2.30 | 0.20 | 0.15 | - | - | | BS-IV | 1.00 | 0.10 | 0.08 | - | - | | Euro-VI | 1.00 | 0.10 | 0.06 | - | 0.005 | **Table: Diesel Emission Norms (g/km)** | Norm | CO | HC | NOx | HC+NOx | PM | |:---------|:-------|:-------|:-------|:-------|:-------| | BS-III | 0.64 | - | 0.50 | 0.56 | 0.05 | | BS-IV | 0.50 | - | 0.25 | 0.30 | 0.025 | | Euro-VI | 0.50 | - | 0.06 | 0.17 | 0.005 | - **Timeline:** GoI planned for BS-VI vehicles from April 2018 and a shift from BS-IV to BS-VI by 2020, bypassing BS-V. ##### EURO Norms (Vehicular Exhaust) - Refers to permissible emission levels implemented in Europe, known as Bharat Norms in India. **Table: EURO Norms (g/km)** | S.No. | Unit | Previous | Bharat 1 | Bharat 2 | Bharat 3 | Bharat 4 | |:------|:--------|:---------|:---------|:---------|:---------|:---------| | CO | Gm/km | 4.34 | 2.75 | 2.20 | 2.30 | 1.0 | | HC+NOx| Gm/km | 1.50 | 0.97 | 0.50 | (0.20)(0.15) | (0.10)(0.08) | ##### Pollution Under Control (PUC) - **Purpose:** Existing system of periodic inspection and maintenance, a mandatory requirement for all categories of on-road vehicles. - **Certificate:** A PUC certificate is issued upon conformity to emissions standards. - **Tests:** - Idle test for gasoline vehicles. - Free acceleration smoke test for diesel vehicles. #### India's Promise to COP26 (Glasgow Pact) - **Emissions Intensity:** India committed to reducing emissions intensity of its GDP by 45% by 2030. - **Net-Zero:** Updated NDC aims for net-zero emissions by 2070. - **Clean Energy:** New framework (2021-2030) for cleaner energy, increasing green jobs, boosting manufacturing of low-emission products (EVs, super-efficient appliances), and promoting innovative technologies (green hydrogen). - **Adaptation:** Stronger adaptation targets, enhancing investments in development programs for climate-vulnerable sectors (agriculture, water resources, health, disaster management, Himalayas, coastal regions). - **Technology:** Building capacities for quick diffusion of cutting-edge climate technology. #### Conference of the Parties (COP) - **Definition:** The world's biggest climate meeting, e.g., COP 28 in Dubai (Nov 30 - Dec 12, 2023). - **UNFCCC:** United Nations Framework Convention on Climate Change, involving 198 countries/parties. - **Goal:** Aims to stabilize greenhouse gas concentrations at a level that prevents dangerous anthropogenic (human-induced) interference with the climate system.