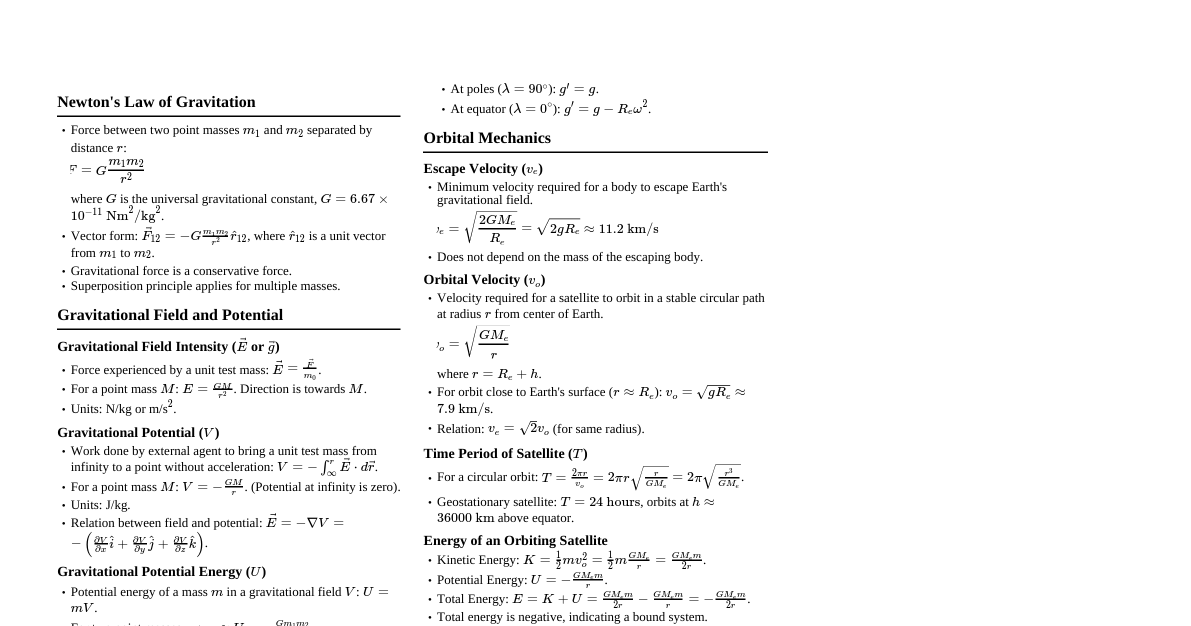
Mathematics: Calculus & Algebra Differentiation Formulas $\frac{d}{dx}(x^n) = nx^{n-1}$ $\frac{d}{dx}(e^x) = e^x$ $\frac{d}{dx}(\ln x) = \frac{1}{x}$ $\frac{d}{dx}(\sin x) = \cos x$ $\frac{d}{dx}(\cos x) = -\sin x$ Product Rule: $\frac{d}{dx}(uv) = u\frac{dv}{dx} + v\frac{du}{dx}$ Quotient Rule: $\frac{d}{dx}\left(\frac{u}{v}\right) = \frac{v\frac{du}{dx} - u\frac{dv}{dx}}{v^2}$ Integration Formulas $\int x^n dx = \frac{x^{n+1}}{n+1} + C \quad (n \neq -1)$ $\int \frac{1}{x} dx = \ln|x| + C$ $\int e^x dx = e^x + C$ $\int \sin x dx = -\cos x + C$ $\int \cos x dx = \sin x + C$ Integration by Parts: $\int u dv = uv - \int v du$ Quadratic Equations Standard form: $ax^2 + bx + c = 0$ Roots: $x = \frac{-b \pm \sqrt{b^2 - 4ac}}{2a}$ Discriminant $\Delta = b^2 - 4ac$: $\Delta > 0$: Two distinct real roots $\Delta = 0$: Two equal real roots $\Delta Sum of roots: $\alpha + \beta = -\frac{b}{a}$ Product of roots: $\alpha \beta = \frac{c}{a}$ Sequences and Series Arithmetic Progression (AP): $a_n = a + (n-1)d$ $S_n = \frac{n}{2}(2a + (n-1)d) = \frac{n}{2}(a + a_n)$ Geometric Progression (GP): $a_n = ar^{n-1}$ $S_n = \frac{a(r^n - 1)}{r-1}$ for $r \neq 1$ $S_\infty = \frac{a}{1-r}$ for $|r| Physics: Mechanics & Electromagnetism Kinematics $v = u + at$ $s = ut + \frac{1}{2}at^2$ $v^2 = u^2 + 2as$ $s_n^{th} = u + \frac{a}{2}(2n - 1)$ Newton's Laws of Motion First Law: Inertia Second Law: $\vec{F} = m\vec{a}$ Third Law: Action-Reaction pairs Momentum: $\vec{p} = m\vec{v}$ Impulse: $\vec{J} = \Delta\vec{p} = \vec{F}_{avg}\Delta t$ Work, Energy, and Power Work Done: $W = \vec{F} \cdot \vec{d} = Fd\cos\theta$ Kinetic Energy: $KE = \frac{1}{2}mv^2$ Potential Energy (Gravitational): $PE = mgh$ Power: $P = \frac{W}{t} = \vec{F} \cdot \vec{v}$ Work-Energy Theorem: $W_{net} = \Delta KE$ Electrostatics Coulomb's Law: $F = k \frac{|q_1 q_2|}{r^2}$, where $k = \frac{1}{4\pi\epsilon_0}$ Electric Field due to point charge: $E = k \frac{|q|}{r^2}$ Electric Potential due to point charge: $V = k \frac{q}{r}$ Electric Potential Energy: $U = k \frac{q_1 q_2}{r}$ Capacitance of parallel plate capacitor: $C = \frac{\epsilon_0 A}{d}$ Current Electricity Ohm's Law: $V = IR$ Resistance in series: $R_{eq} = R_1 + R_2 + ...$ Resistance in parallel: $\frac{1}{R_{eq}} = \frac{1}{R_1} + \frac{1}{R_2} + ...$ Joule's Heating: $H = I^2Rt = VIt = \frac{V^2}{R}t$ Kirchhoff's Laws: Junction Rule: $\sum I_{in} = \sum I_{out}$ Loop Rule: $\sum \Delta V = 0$ Chemistry: Physical & Organic Atomic Structure Bohr's Radius: $r_n = 0.529 \frac{n^2}{Z}$ Å Energy of $n^{th}$ orbit: $E_n = -13.6 \frac{Z^2}{n^2}$ eV/atom De Broglie wavelength: $\lambda = \frac{h}{mv}$ Heisenberg's Uncertainty Principle: $\Delta x \cdot \Delta p \ge \frac{h}{4\pi}$ Chemical Bonding & Molecular Structure Hybridization: Count steric number (lone pairs + sigma bonds) 2: $sp$ 3: $sp^2$ 4: $sp^3$ 5: $sp^3d$ 6: $sp^3d^2$ Dipole Moment: $\mu = q \times d$ Bond Order (from MOT): $\frac{1}{2}(\text{No. of electrons in bonding MO} - \text{No. of electrons in anti-bonding MO})$ States of Matter Ideal Gas Equation: $PV = nRT$ Dalton's Law of Partial Pressures: $P_{total} = P_1 + P_2 + ...$ Graham's Law of Diffusion: $\frac{r_1}{r_2} = \sqrt{\frac{M_2}{M_1}}$ Van der Waals Equation: $\left(P + \frac{an^2}{V^2}\right)(V - nb) = nRT$ Thermodynamics First Law: $\Delta U = Q + W$ Work done by gas: $W = -P_{ext}\Delta V$ (for expansion) Enthalpy: $H = U + PV$ Gibbs Free Energy: $\Delta G = \Delta H - T\Delta S$ $\Delta G = -RT \ln K_{eq}$ Hydrocarbons (Organic Chemistry) Alkanes: Saturated, $C_n H_{2n+2}$, substitution reactions Alkenes: Unsaturated, $C_n H_{2n}$, addition reactions (Markovnikov's rule) Alkynes: Unsaturated, $C_n H_{2n-2}$, addition reactions Aromatic Compounds (Benzene): Electrophilic substitution (e.g., nitration, halogenation, Friedel-Crafts) General Organic Chemistry (GOC) Inductive Effect: Permanent, distance-dependent electron displacement. $+I$ groups: alkyl groups $-I$ groups: $-NO_2, -CN, -COOH, -X$ Resonance Effect: Delocalization of $\pi$ electrons. $+R$ groups: $-OH, -OR, -NH_2, -X$ (electron-donating) $-R$ groups: $-NO_2, -CN, -CHO, -COOH$ (electron-withdrawing) Acidity: Favored by electron-withdrawing groups (stabilize conjugate base) Basicity: Favored by electron-donating groups (increase electron density on N/O)