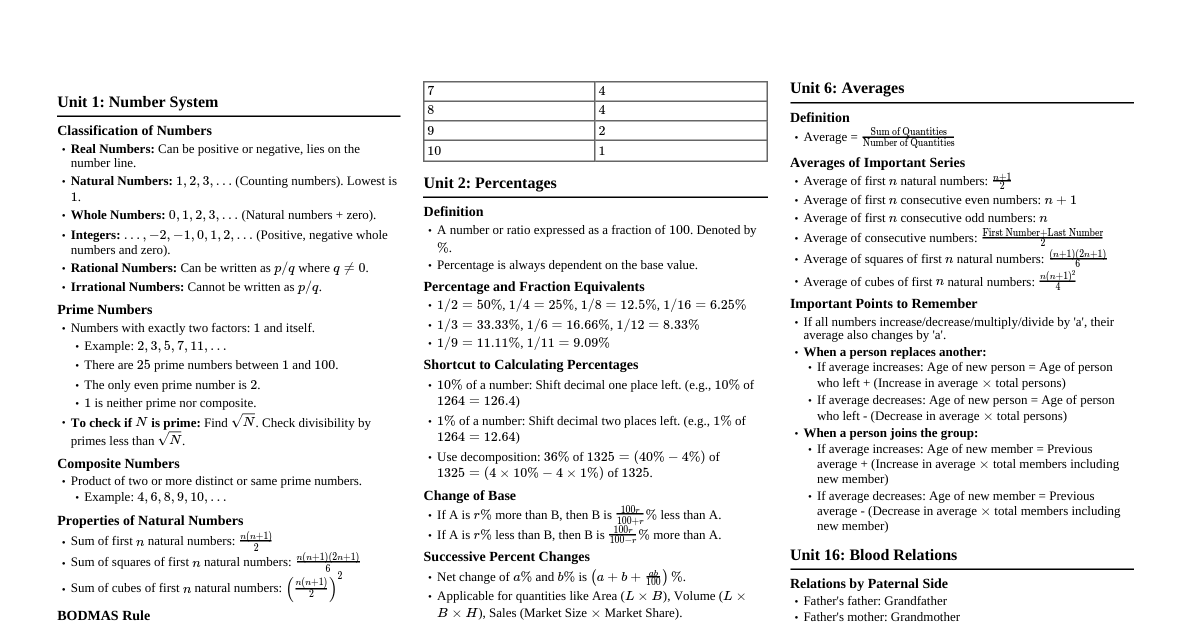
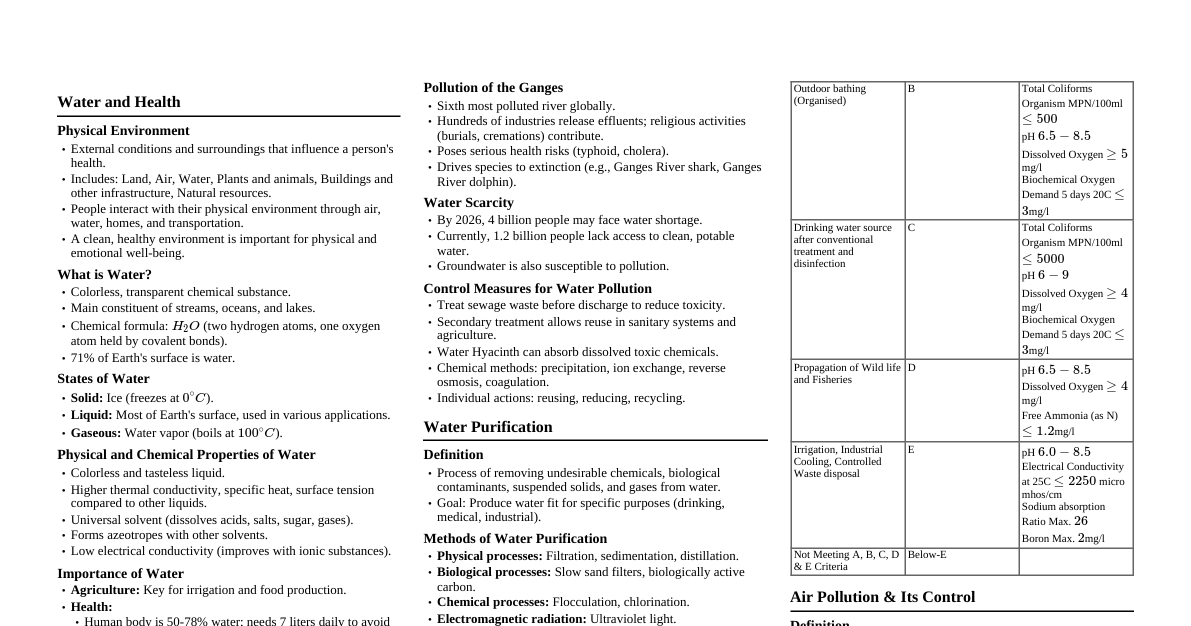
Dielectric Strength: Core Concepts Definition: Maximum electric field a material withstands before breakdown. Importance: Critical for electrical insulation design. Breakdown: Material becomes conductive, leading to failure. Internal Factors Affecting Dielectric Strength 1. Material Properties Chemical Composition & Purity: Composition: Types of atoms and their bonding (e.g., strong covalent/ionic bonds often imply higher intrinsic strength). Presence of polar groups affects dielectric constant and losses. Purity: Impurities (e.g., moisture, dirt, metallic particles, voids) create localized stress points, reducing strength significantly. Homogeneity: Uniform materials tend to have higher and more consistent dielectric strength. Physical State: Solids > Liquids > Gases in general strength. Gases/Liquids offer self-healing. Thickness: Strength generally decreases as thickness increases (due to higher defect probability). Approximate: $E_b \propto d^{-n}$. Mechanical Properties: Hardness: Harder materials (e.g., ceramics, glass) are less prone to physical damage and penetration by contaminants, which can maintain insulation integrity. Brittleness: Brittle materials (e.g., glass, porcelain) are susceptible to cracking under mechanical or thermal stress, creating weak points for breakdown. Flexibility (e.g., rubber, some plastics) can be advantageous in dynamic applications. Tensile Strength/Elasticity: Materials with good mechanical strength and elasticity can better withstand electromechanical stresses and thermal expansion/contraction without damage. 2. Temperature General Trend: Strength typically decreases with increasing temperature. Reason: Increased molecular agitation and mobility at higher temperatures can make it easier for electrons to gain sufficient energy for ionization and breakdown. Dipolar Relaxation: At certain temperatures and frequencies, polar molecules in the dielectric may align with the electric field, causing energy loss (dielectric heating) which can contribute to thermal breakdown, especially under AC fields. Thermal Breakdown: High temperatures can lead to thermal runaway if heat generation exceeds dissipation. External Factors Affecting Dielectric Strength 1. Environmental Conditions Moisture Content (Humidity): Significantly reduces strength due to increased conductivity and partial discharges. Pressure (Gases/Liquids): Gases: Strength increases with pressure (Paschen's Law). Liquids: Moderate effect, can suppress bubble formation. Contamination & Surface Conditions: Rough surfaces, dust, dirt, oil $\rightarrow$ Localized field enhancements, conductive paths, reduced flashover voltage. Aging (UV, ozone) $\rightarrow$ Degradation over time. 2. Applied Electrical Stress Type of Voltage: DC Voltage: Highest strength (uniform stress). AC Voltage: Lower strength (continuous stress, peak voltage critical). Impulse Voltage: Lowest strength (short duration, rapid energy deposition). Duration of Voltage: Longer duration $\rightarrow$ Lower strength (allows degradation to develop). Shorter duration $\rightarrow$ Higher strength. Electrode Configuration: Uniform fields (parallel plates) $\rightarrow$ Highest strength. Non-uniform fields (sharp points) $\rightarrow$ Lower strength due to localized field enhancement. Dipolar Relaxation Definition: The process by which permanent or induced dipoles in a dielectric material reorient themselves in response to an applied electric field. Frequency Dependence: Occurs over a range of frequencies, leading to energy absorption (dielectric loss). Impact on Strength: High dielectric loss due to dipolar relaxation can lead to significant heating within the material, contributing to thermal breakdown, especially under AC fields. Temperature Dependence: Relaxation times are temperature-dependent; increased temperature can shift the relaxation frequency. Dielectric Constant ($\epsilon_r$) Definition: A measure of a material's ability to store electrical energy in an electric field. Ratio of capacitance with dielectric to capacitance with vacuum. Field Distribution: In multi-layer dielectric systems, the electric field distributes inversely proportional to the dielectric constant. Materials with lower $\epsilon_r$ experience higher electric stress. Field Enhancement: High $\epsilon_r$ can cause field enhancement at interfaces or points of discontinuity if not properly designed, leading to localized stress and potential breakdown. Relationship to Polarization: Higher $\epsilon_r$ generally means the material can be more easily polarized, storing more energy. Breakdown Mechanisms Intrinsic Breakdown: Theoretical maximum strength, limited by the material's atomic structure. Occurs at very high fields, often at low temperatures. Thermal Breakdown: Occurs when dielectric losses generate heat faster than it can dissipate, leading to a thermal runaway and localized melting/carbonization. Electromechanical Breakdown: High electric fields can cause forces that deform the material, eventually leading to a reduction in thickness and breakdown. Discharge Breakdown: Caused by partial discharges (PD) in voids or on surfaces, leading to erosion and eventual complete breakdown. Tracking and Erosion: Formation of conductive paths on the surface of an insulator due to voltage stress and environmental factors. Breakdown Mechanisms in Specific Media 1. Breakdown in Gases Ionization: Free electrons present in the gas gain energy from the electric field, collide with gas molecules, and ionize them, creating more electrons and positive ions. Townsend Mechanism: A cumulative process where initial electrons create secondary electrons through impact ionization. Positive ions also contribute by releasing electrons from the cathode. Requires a critical field to sustain the discharge. Streamer Mechanism: In highly non-uniform fields or at higher pressures, a rapid avalanche of electrons forms a "streamer" (plasma channel) that propagates across the gap, leading to breakdown. Paschen's Law: Describes the relationship between breakdown voltage, pressure ($p$), and electrode gap distance ($d$) for uniform fields: $V_b = f(pd)$. There is an optimum $pd$ for minimum breakdown voltage. 2. Breakdown in Liquids Electronic Breakdown: Similar to gases, free electrons gain energy and cause ionization. However, liquids have higher density, so electron mean free path is very short, requiring much higher fields. Cavitation/Bubble Breakdown: Micro-bubbles (due to impurities, gas evolution, or localized heating) can form in the liquid. Gases within these bubbles have lower dielectric strength than the liquid, leading to partial discharges inside the bubbles. These discharges erode the liquid and expand the bubble, eventually bridging the gap and causing full breakdown. Suspended Particle Breakdown: Conducting particles (e.g., dust, metallic impurities) suspended in the liquid can align along the electric field lines, forming "bridges" that reduce the effective insulating distance and lead to breakdown. Particles can also cause localized field enhancement. Electro-convection Breakdown: In some liquids, impurities or space charges can lead to liquid motion (electro-convection), which can transport charge and initiate breakdown. Common Insulator Materials & Dielectric Constants ($\epsilon_r$) 1. Inorganic Solids Mica: $\epsilon_r \approx 5-7$. Excellent dielectric strength, high temperature resistance, low loss. Used in capacitors, high-voltage insulation. Glass: $\epsilon_r \approx 3.7-10$ (depending on type). High resistivity, good chemical stability. Used in bushings, insulators, lamp envelopes. Porcelain: $\epsilon_r \approx 6-8$. High mechanical strength, good weather resistance, low cost. Widely used for outdoor insulators (e.g., power lines). Asbestos: (Historical, use now severely restricted due to health hazards) $\epsilon_r \approx 3-5$. High heat resistance, good insulating properties. 2. Organic Solids Paper (impregnated): $\epsilon_r \approx 3-4.5$. Used in cables, capacitors, transformers. Good mechanical strength when layered. Rubber: $\epsilon_r \approx 2.5-4$. Flexible, good water resistance. Used for cable insulation, protective equipment. Plastics: Polyethylene (PE): $\epsilon_r \approx 2.2-2.4$. Excellent insulator, low loss. Cable insulation. Polyvinyl Chloride (PVC): $\epsilon_r \approx 3-6$. Flexible, fire-retardant. Cable sheathing, low-voltage insulation. Polypropylene (PP): $\epsilon_r \approx 2.2-2.6$. Capacitors, flexible films. Polyester (PET): $\epsilon_r \approx 3-3.3$. Film capacitors, slot liners. Bakelite (Phenolic Resin): $\epsilon_r \approx 5-7$. Hard, rigid, good heat resistance. Used for switchgear, terminal boards. 3. Resins and Varnishes Epoxy Resins: $\epsilon_r \approx 3-5$. Potting, encapsulation, PCBs. Excellent adhesion, strength. Silicone Resins: $\epsilon_r \approx 2.5-3.5$. High temperature, water repellent coatings. 4. Liquid Insulators Transformer Oil (Mineral Oil): $\epsilon_r \approx 2.2-2.4$. Widely used in transformers, switchgear, and some cables. Provides both insulation and cooling due to good thermal conductivity. Self-healing properties after minor breakdowns, as the oil can flow to fill the breakdown path. Purity (especially moisture and gas content) significantly affects its dielectric strength. Water content above a few ppm drastically reduces strength. Synthetic Esters: $\epsilon_r \approx 3.2-3.4$. Biodegradable alternatives to mineral oil, often with higher fire points and better moisture tolerance. 5. Gaseous Insulators Air: $\epsilon_r \approx 1.00059$. Most common, cheapest, and environmentally benign insulator. Dielectric strength is relatively low ($\approx 3 \text{ kV/mm}$ at STP), and highly dependent on pressure, temperature, and humidity. Used in open-air switchgear, overhead transmission lines, and as general spacing in electrical equipment. Sulfur Hexafluoride ($SF_6$): $\epsilon_r \approx 1.002$. Excellent dielectric strength (2-3 times that of air at atmospheric pressure). High electronegativity, meaning it readily captures free electrons, preventing avalanche breakdown. Used extensively in gas-insulated switchgear (GIS), circuit breakers, and high-voltage applications where space is limited. Environmentally potent greenhouse gas (Global Warming Potential $\approx 23,500$ times that of $CO_2$), leading to stringent regulations and research into alternatives. Nitrogen ($N_2$): $\epsilon_r \approx 1.00055$. Dielectric strength is slightly better than air, but significantly lower than $SF_6$. Non-flammable, non-toxic, and environmentally friendly. Can be used as an insulating medium, sometimes mixed with $SF_6$ or in applications where a moderate dielectric strength is acceptable. Increasingly considered as an $SF_6$ alternative or in $SF_6$/$N_2$ mixtures for environmental reasons. Hydrogen ($H_2$): $\epsilon_r \approx 1.00026$. Historically used where good cooling and high thermal conductivity were needed (e.g., large generators, synchronous condensers). Relatively low dielectric strength compared to $SF_6$. Flammable, requiring careful handling and sealed systems. Ageing of Insulators Definition: The irreversible degradation of insulation properties over time due to various stresses, leading to a reduction in dielectric strength and eventual failure. Types of Stresses (Multi-stress Ageing): Electrical Stress: Continuous voltage, partial discharges, transient overvoltages. Thermal Stress: Operating temperature, temperature cycles, localized hotspots. Mechanical Stress: Vibrations, thermal expansion/contraction, electromagnetic forces. Environmental Stress: Moisture, UV radiation, chemical contaminants, pollution. Consequences: Reduction in dielectric strength and resistivity. Increase in dielectric losses. Physical changes: embrittlement, cracking, erosion, carbonization. Increased susceptibility to breakdown. Monitoring: Regular testing (e.g., partial discharge, dielectric loss, insulation resistance, dissolved gas analysis for oils) is crucial to assess insulation health and predict remaining life.