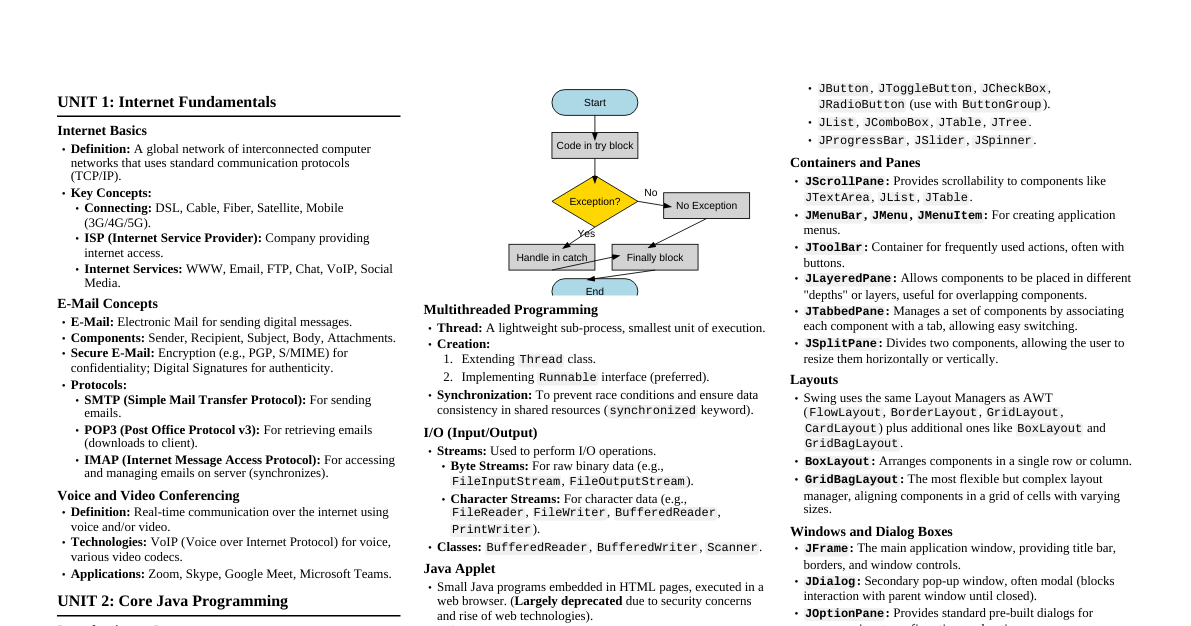
### Introduction to Spectroscopy Spectroscopy is the study of the interaction between matter and electromagnetic radiation. It provides information about the properties, composition, and structure of molecules. Molecules are too small to be seen directly, so spectroscopy is crucial for understanding their structure. #### Interaction of Radiation with Matter Radiation is a form of energy that can be absorbed or transmitted. The interaction involves transitions between different energy levels within atoms or molecules. These energy levels are associated with: - Electronic states - Vibrations between atoms - Rotation of groups of atoms The absorption or emission of radiant energy causes transitions between these quantized energy levels. Analyzing the wavelength or frequency of absorbed/emitted radiation reveals information about the molecule's identity. #### Types of Spectra 1. **Emission Spectra:** Produced when atoms release energy. It is a discontinuous spectrum with distinct wavelengths. Helps identify the elements in a substance. 2. **Absorption Spectra:** Produced when atoms absorb energy. It is a continuous spectrum with dark lines or gaps where energy was absorbed. Helps determine the quantity of substances in a sample. Emission or absorption spectra can be further classified: - **Atomic Spectra:** Obtained when atoms interact with electromagnetic radiation. Involves electron transitions between electronic energy levels. Appears as sharp lines, characteristic "fingerprints" of atoms. - **Molecular Spectra:** Deals with interactions of electromagnetic radiation with molecules. Involves transitions between electronic, vibrational, and rotational energy levels. #### Major Types of Spectroscopy Based on the nature of radiation absorbed or emitted: - Ultraviolet/Visible (UV-Vis) Spectroscopy - Infrared (IR) Spectroscopy - Nuclear Magnetic Resonance (NMR) Spectroscopy - Mass Spectrometry (often grouped, though not strictly an interaction with EM radiation in the same way) - Others: Atomic Absorption, Raman, X-ray, Fluorescence, Mössbauer, Surface Plasmon Resonance (SPR). ### UV-Vis Spectroscopy Also known as Electronic Spectroscopy, it involves the promotion of electrons (sigma, pi, n electrons) from ground to higher energy states. #### Applications - Measures conjugated double bonds and aromatic conjugation. - Distinguishes between conjugated and non-conjugated systems. - Distinguishes homoannular and heteroannular conjugated dienes. #### Spectral Region - UV: 185–400 nm - Visible: 400–700 nm - Near Infrared (NIR): 700–1100 nm Most commercial spectrophotometers cover 185 to 900 nm. #### Types of Energy Changes in Molecules (Relevant to UV-Vis) The total energy of a molecule is the sum of translational, rotational, vibrational, and electronic energy. UV-Vis primarily deals with electronic transitions. #### Beer-Lambert's Law States that when monochromatic radiation passes through an absorbing solution, the rate of decrease in intensity with thickness is directly proportional to the intensity of incident radiation and the concentration of the solution. $$A = \epsilon c l$$ Where: - $A$ = Absorbance - $\epsilon$ = Molar absorption coefficient (molar absorptivity or molar extinction coefficient) - $c$ = Concentration (mol/L) - $l$ = Path length (cm) The molar absorption coefficient ($\epsilon$) is the absorbance of a solution of unit concentration and unit path length. Its unit is Conc$^{-1}$ length$^{-1}$. #### Conditions for Beer-Lambert's Law - Light must be monochromatic. - Concentrations must be low. - Solution must not be fluorescent or heterogeneous. - Solute must not undergo photochemical transformations. - Solute must not undertake variable associations with the solvent. #### Types of Electronic Transitions In organic molecules, common electronic transitions include: - $\sigma \rightarrow \sigma^*$ (high energy, typically in vacuum UV) - $n \rightarrow \sigma^*$ (occurs in compounds with lone pairs, e.g., alcohols, ethers) - $\pi \rightarrow \pi^*$ (occurs in compounds with double/triple bonds, e.g., alkenes, alkynes, aromatics) - $n \rightarrow \pi^*$ (occurs in compounds with lone pairs and double/triple bonds, e.g., carbonyls) #### Chromophore A chromophore is an isolated covalently bonded group that shows characteristic absorption in the UV-Vis region (e.g., C=C, C=O, N=N). A chromogene is a carbon skeleton transparent in UV-Vis with one or several chromophores attached. #### Auxochrome An auxochrome is a group that does not absorb in the UV-Vis region itself but shifts the absorption band of a chromophore towards longer wavelengths (bathochromic shift) and often increases its intensity (hyperchromic effect). Examples: -OH, -OR, -NH$_2$, -NHR, -SH. #### Factors Affecting $\lambda_{max}$ (Shifts) 1. **Bathochromic Effect (Red Shift):** Shift of $\lambda_{max}$ to longer wavelengths due to auxochromes or solvent changes (e.g., $n \rightarrow \pi^*$ transition of carbonyls). 2. **Hypsochromic Effect (Blue Shift):** Shift of $\lambda_{max}$ to shorter wavelengths due to loss of conjugation or change in solvent polarity. 3. **Hyperchromic Effect:** Increase in the intensity of absorption ($\epsilon_{max}$). Often caused by introducing an auxochrome. 4. **Hypochromic Effect:** Decrease in the intensity of absorption ($\epsilon_{max}$). Caused by distortion of molecular geometry. #### Other Factors Affecting $\lambda_{max}$ - **Solvent:** Choice of solvent can shift peaks due to interaction with the chromophore's excited state. Ethanol solutions often give longer wavelength maxima than hexane. - **Concentration:** Proportional to intensity at low concentrations. High concentrations can lead to molecular interactions (e.g., polymerization) affecting peak position and shape, impacting Beer's Law linearity. - **pH:** Significant impact, primarily by shifting equilibrium between different chemical forms of an analyte. Buffers can maintain pH, but must be transparent in the measured wavelength range. - **Temperature:** Affects absorption through solvent expansion/contraction, shifts in chemical equilibria, changes in reaction rates, and variation in refractive index. #### Woodward-Fieser Rules Empirical rules used to predict the $\lambda_{max}$ for conjugated dienes and $\alpha,\beta$-unsaturated carbonyl compounds. They involve a base value for a parent system and additive increments for substituents, rings, and exocyclic double bonds. ### IR Spectroscopy Infrared (IR) spectroscopy studies the interaction of IR radiation with matter, causing bond vibrations and providing information about molecular structure and functional groups. Also called vibrational-rotational spectroscopy. #### IR Regions - **Near IR:** 0.8 - 2.5 µm (12500 - 4000 cm$^{-1}$) - **Middle IR:** 2.5 - 15 µm (4000 - 667 cm$^{-1}$) - *Main region for analytical purposes.* - **Far IR:** 15 - 200 µm (667 - 50 cm$^{-1}$) #### Basic Principles - Bonds in a molecule are not rigid; they vibrate at a natural frequency. - When IR radiation matches the natural frequency, absorption occurs, exciting the molecule and producing a peak. - IR spectra are vibrational-rotational spectra, as each vibrational level has closely spaced rotational levels. - Different bonds and functional groups absorb at characteristic frequencies, acting as a "fingerprint" for a molecule. - **Group Frequency Region:** Higher frequency region where characteristic functional group absorptions are found. - **Fingerprint Region:** Lower frequency region (below ~1500 cm$^{-1}$) with complex, unique patterns specific to a molecule. #### IR Activity (Selection Rules) Only bonds with a **change in dipole moment** during vibration will absorb IR radiation (infrared active transitions). - **Infrared Active:** C=O, N-H, O-H (polar bonds). - **Infrared Inactive:** Symmetrical C=C bonds in alkenes or C≡C bonds in alkynes (no net change in dipole moment). #### Types of Vibrations 1. **Stretching Vibrations:** Change in bond length along the bond axis. - Symmetric stretching - Asymmetric stretching 2. **Bending Vibrations:** Change in the angle between bonds. - **In-plane bending:** - Scissoring (two atoms move towards each other) - Rocking (two atoms move in the same direction) - **Out-of-plane bending:** - Wagging (two atoms move up or down relative to the plane) - Twisting (one atom moves up, the other down relative to the plane) #### Hooke's Law Used to calculate the stretching vibrational frequency ($\nu$) of a bond, modeled as a harmonic oscillator: $$\nu = \frac{1}{2\pi c} \sqrt{\frac{k}{\mu}}$$ Where: - $\nu$ = Vibrational frequency (cm$^{-1}$) - $c$ = Speed of light - $k$ = Force constant (bond strength) - $\mu$ = Reduced mass of the diatomic molecule ($\mu = \frac{m_1 m_2}{m_1 + m_2}$) #### Factors Affecting Vibrational Frequency - **Bond Strength (k):** Stronger bonds have higher frequencies (e.g., C=C > C-C). - **Reduced Mass ($\mu$):** Lighter atoms (smaller reduced mass) have higher frequencies (e.g., O-H > C-C). - **Hydrogen Bonding:** Causes characteristic shifts in O-H and N-H stretching frequencies. #### Identification of Functional Groups - **C-C:** Alkanes have C-H stretches ~2850-2960 cm$^{-1}$ and C-C stretches in fingerprint region. - **C=C:** Alkenes have C=C stretch ~1620-1680 cm$^{-1}$ (if IR active) and =C-H stretches ~3010-3100 cm$^{-1}$. - **-OH:** Alcohols have broad O-H stretch ~3200-3600 cm$^{-1}$ (hydrogen bonded) or sharp ~3600-3650 cm$^{-1}$ (free). - **-NH$_2$:** Primary amines have two N-H stretches (symmetric and asymmetric) ~3300-3500 cm$^{-1}$. - **-C=O:** Carbonyl compounds have strong C=O stretch ~1650-1780 cm$^{-1}$, position depends on conjugation, ring strain, etc. ### NMR Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy involves the interaction between an oscillating magnetic field of electromagnetic radiation (radio waves) and the magnetic energy of certain nuclei (e.g., $^1$H, $^{13}$C) when placed in an external static magnetic field. The sample absorbs radiation at different frequencies, depending on the type of nuclei and their chemical environment. #### Basic Principles - **Nuclear Spin:** Nuclei with an odd mass number or odd atomic number possess nuclear spin and act like tiny bar magnets. (e.g., $^1$H, $^{13}$C). - **External Magnetic Field ($B_0$):** When placed in $B_0$, these nuclear magnets align either with (lower energy, $\alpha$-spin state) or against (higher energy, $\beta$-spin state) the applied field. - **Resonance:** When radiofrequency (RF) energy matching the energy difference between these spin states is applied, nuclei in the $\alpha$-state flip to the $\beta$-state, absorbing energy. This phenomenon is called resonance. #### Chemical Shift - **Shielding/Deshielding:** The electron density around a nucleus generates a secondary magnetic field that can either oppose (shielding) or reinforce (deshielding) the applied external magnetic field ($B_0$). - **Shielding:** Electrons circulate, creating an induced magnetic field that opposes $B_0$. The nucleus experiences a weaker effective magnetic field, requiring a lower applied frequency to achieve resonance. This shifts the absorption **upfield** (to lower chemical shift values). - **Deshielding:** Electron-withdrawing groups or certain molecular geometries can reduce electron density around a nucleus or create induced fields that reinforce $B_0$. The nucleus experiences a stronger effective magnetic field, requiring a higher applied frequency to achieve resonance. This shifts the absorption **downfield** (to higher chemical shift values). - **Definition:** Chemical shift is the difference in the resonance frequency of a particular nucleus compared to a standard reference compound, measured in parts per million (ppm). It reflects the electronic environment of the nucleus. #### Measurement of Chemical Shift - **Standard Reference:** Tetramethylsilane (TMS, (CH$_3$)$_4$Si) is the universal standard for $^1$H and $^{13}$C NMR. - TMS has 12 equivalent protons (or 4 equivalent carbons), giving a sharp single signal. - Due to silicon's low electronegativity, TMS protons are highly shielded, absorbing at a very high field (designated as 0 ppm). - The chemical shift ($\delta$) is calculated as: $$\delta \text{ (ppm)} = \frac{(\text{nucleus frequency} - \text{TMS frequency})}{\text{spectrometer frequency}} \times 10^6$$ #### Factors Affecting Chemical Shift - **Electronegativity:** More electronegative atoms withdraw electron density, deshielding nearby protons and shifting their signals downfield. - Example: CH$_3$Cl ($\delta \approx 3.0$) vs. CH$_3$F ($\delta \approx 4.2$) - **Hybridization:** Protons on $sp^2$ hybridized carbons are generally more deshielded than those on $sp^3$ carbons due to anisotropic effects. - Example: Alkene protons ($\delta \approx 4.5-6.0$) vs. alkane protons ($\delta \approx 0.9-1.7$) - **Anisotropic Effects:** Magnetic fields induced by $\pi$-electron systems (e.g., alkenes, alkynes, aromatics, carbonyls) can either shield or deshield nearby protons depending on their position relative to the $\pi$-system. - **Benzene:** Protons are strongly deshielded ($\delta \approx 7.2$) because they are in the deshielding region of the induced ring current. - **Alkynes:** Acetylenic protons ($\delta \approx 2.0-3.0$) are relatively shielded because they lie in the shielding region of the induced circulation of $\pi$-electrons. - **Hydrogen Bonding:** Protons involved in hydrogen bonding are deshielded, and their chemical shift is temperature and concentration dependent (e.g., -OH, -NH protons). - **Acidic Protons:** Protons on carboxylic acids ($\delta \approx 10-13$) are highly deshielded. #### Structural Elucidation By analyzing the number of signals, their chemical shifts, and splitting patterns (due to spin-spin coupling, not covered in detail here but implied by "structural elucidation"), one can deduce the structure of simple organic compounds. ### Mass Spectrometry Mass Spectrometry (MS) is an analytical technique that measures the mass-to-charge ratio (m/z) of ions. It is used to determine the molecular weight, elemental composition, and structural information of molecules. Unlike other spectroscopies, MS typically does not involve interaction with electromagnetic radiation in the same way (it's a measure of mass). #### Basic Principle 1. **Ionization:** Molecules are converted into ions (usually cations) in the gas phase. 2. **Acceleration:** Ions are accelerated through an electric field. 3. **Deflection:** Ions are passed through a magnetic field or electric field, which deflects them based on their m/z ratio. Lighter ions and ions with higher charge are deflected more. 4. **Detection:** A detector records the abundance of each ion at different m/z ratios, generating a mass spectrum. #### Key Information from Mass Spectrum - **Molecular Ion Peak (M$^+$):** The peak corresponding to the intact molecule minus one electron (or plus a proton, depending on ionization method). It gives the molecular weight. - **Base Peak:** The most intense peak in the spectrum, assigned 100% relative abundance. - **Fragment Ions:** Peaks at lower m/z values than M$^+$ due to fragmentation of the molecular ion. These fragments provide structural information. - **Isotope Peaks:** Small peaks appearing at M+1, M+2, etc., due to the presence of naturally occurring heavier isotopes (e.g., $^{13}$C, $^2$H, $^{37}$Cl, $^{81}$Br). #### Common Ionization Techniques - **Electron Ionization (EI):** High-energy electrons bombard the sample, removing an electron and forming a radical cation (M$^+$). Leads to significant fragmentation. - **Chemical Ionization (CI):** Sample ions are formed by reaction with a reagent gas plasma. Produces less fragmentation, often yielding a strong [M+H]$^+$ peak. - **Electrospray Ionization (ESI):** Sample solution is sprayed through a high-voltage needle, creating charged droplets that desolvate to produce ions (often [M+H]$^+$ or [M+Na]$^+$). Good for large, polar, non-volatile molecules. - **Matrix-Assisted Laser Desorption/Ionization (MALDI):** Sample is mixed with a matrix, dried, and then irradiated with a laser. The matrix absorbs the laser energy, transferring charge to the analyte. Excellent for very large biomolecules (proteins, polymers). #### Applications - Determination of molecular weight. - Identification of unknown compounds. - Quantification of compounds in a mixture. - Structural elucidation through fragmentation patterns. - Analysis of biomolecules (proteins, peptides, DNA). ### Surface Plasmon Resonance (SPR) Surface Plasmon Resonance (SPR) is a label-free optical technique used to monitor binding events between molecules in real-time. It relies on the phenomenon of surface plasmons—electron oscillations at a metal-dielectric interface. #### Basic Principle 1. **Surface Plasmons:** When light (p-polarized) strikes a thin metal film (usually gold or silver) at a specific angle, photons can couple with free electrons at the metal surface, generating surface plasmons. This coupling occurs at a characteristic angle called the SPR angle. 2. **Evanescent Wave:** The light that excites the surface plasmons does not penetrate the metal but generates an evanescent wave that extends a short distance into the dielectric medium (e.g., a buffer solution) above the metal surface. 3. **Refractive Index Sensitivity:** The SPR angle is highly sensitive to changes in the refractive index of the medium immediately adjacent to the metal surface. 4. **Binding Detection:** If one molecule (ligand) is immobilized on the metal surface and another molecule (analyte) binds to it from the solution, the mass accumulates on the surface, changing the local refractive index. This change causes a shift in the SPR angle, which is detected as a change in reflectivity. #### Kretschmann Configuration The most common setup for SPR involves the Kretschmann configuration: - A prism is used to direct light onto a thin metal film coated on its base. - The analyte solution flows over the metal film. - Light is totally internally reflected at the prism-metal interface, and the evanescent wave penetrates the metal and interacts with the sample. - The reflected light intensity is monitored as a function of incident angle or wavelength. A drop in reflectivity indicates SPR. #### Applications - **Kinetic Analysis:** Measures association ($k_a$) and dissociation ($k_d$) rate constants of binding interactions. - **Affinity Determination:** Calculates equilibrium dissociation constant ($K_D$). - **Concentration Measurement:** Quantifies analyte concentration. - **Specificity Studies:** Confirms specific binding. - **Drug Discovery:** Screening for drug candidates, characterizing drug-target interactions. - **Immunosensor Development:** Detecting antibodies/antigens. - **Protein-Protein, Protein-DNA, Protein-Lipid Interactions:** Studying various biomolecular interactions. ### Syllabus Overview This cheatsheet covers fundamental concepts in spectroscopy, including: - **Introduction:** What spectroscopy is, its importance, interaction of radiation with matter, types of spectra (emission/absorption, atomic/molecular). - **UV-Vis Spectroscopy:** Beer-Lambert's law, types of electronic transitions, concept of auxochrome and chromophore, factors affecting $\lambda_{max}$ (shifts, solvent, concentration, pH, temperature), Woodward-Fieser rules for $\lambda_{max}$ calculation in diene systems. - **IR Spectroscopy:** Introduction, types of vibration (stretching, bending), selection rules (dipole moment change), Hooke's law, factors affecting vibrational frequency, identification of functional groups (C-C, C=C, -OH, -NH$_2$, -C=O). - **NMR Spectroscopy:** Basics of NMR, chemical shift, shielding and deshielding effect, factors affecting chemical shift, structural elucidation of simple compounds. - **Mass Spectrometry:** Introduction to principles, ionization techniques (EI, CI, ESI, MALDI), interpretation of mass spectra (molecular ion, fragments, isotopes), applications. - **Surface Plasmon Resonance (SPR):** Principles, Kretschmann configuration, applications in biomolecular interaction analysis.