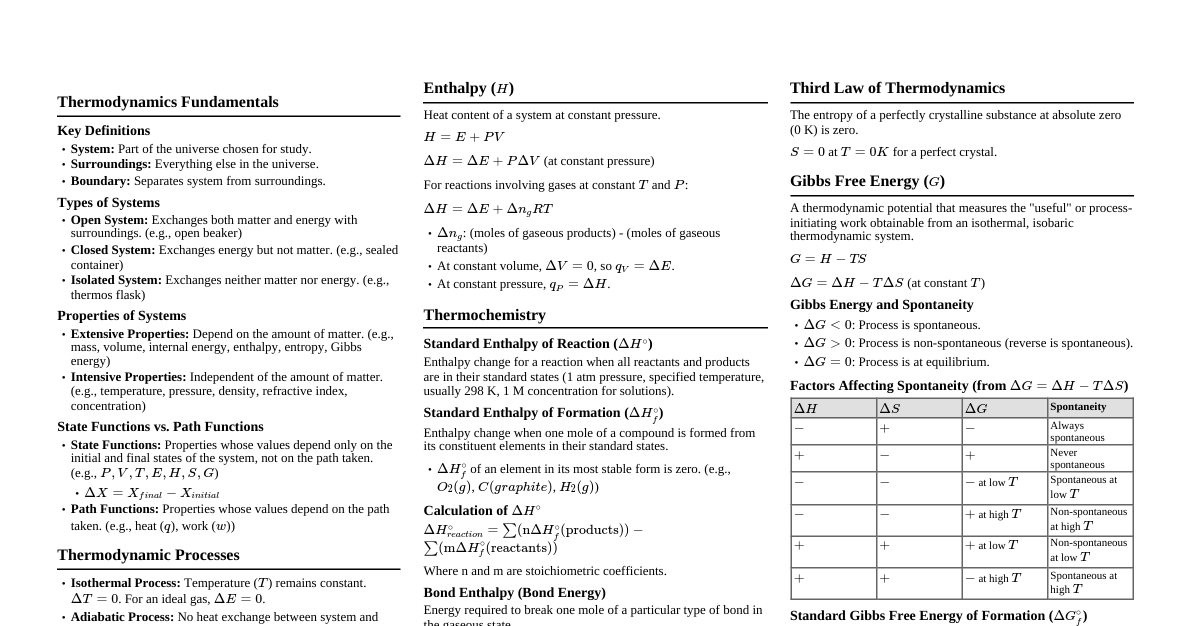
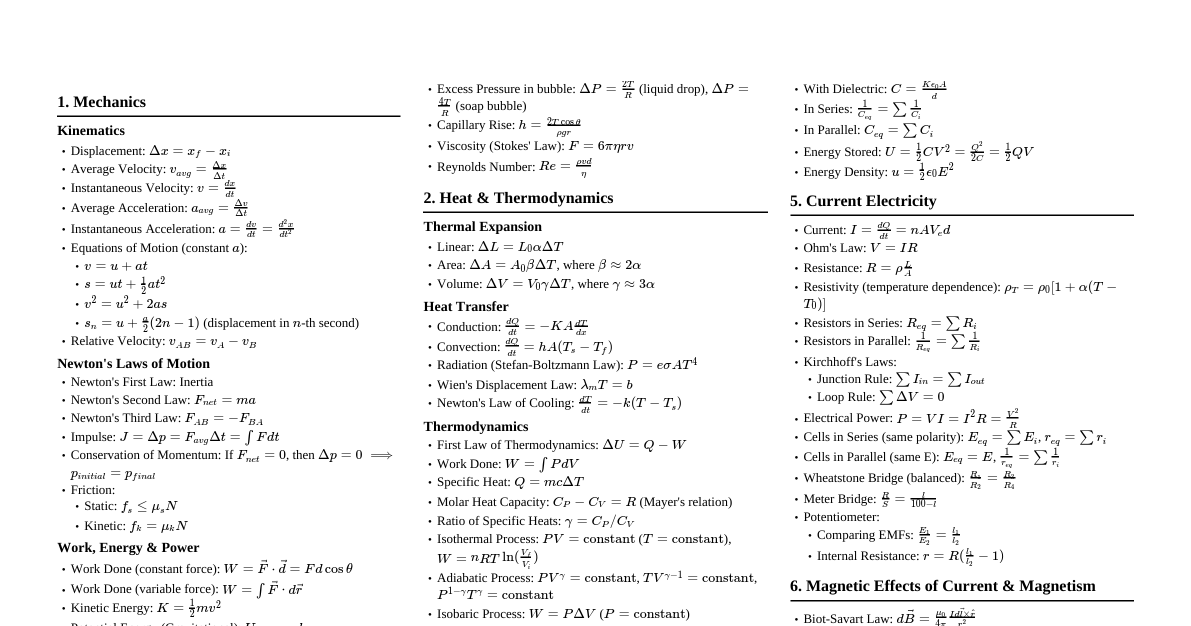
### Thermodynamic Systems & Processes - **System:** A specific part of the universe under consideration. - **Open System:** Exchanges both energy and matter with surroundings (e.g., boiling water in an open pot). - **Closed System:** Exchanges energy but not matter (e.g., boiling water in a closed pot). - **Isolated System:** Exchanges neither energy nor matter (e.g., an ideal thermos flask). - **Surroundings:** Everything external to the system. - **Boundary:** Separates system from surroundings. - **Thermodynamic Variables:** Pressure (P), Volume (V), Temperature (T), Internal Energy (U), Entropy (S). - **Thermodynamic Process:** Change in the state of a system. - **Isothermal Process:** Temperature (T) remains constant. (e.g., melting ice). $PV = \text{constant}$. - **Adiabatic Process:** No heat exchange (Q=0) with surroundings. $PV^\gamma = \text{constant}$. - **Isobaric Process:** Pressure (P) remains constant. (e.g., heating water in an open container). - **Isochoric Process:** Volume (V) remains constant. (e.g., heating gas in a rigid container). - **Cyclic Process:** System returns to its initial state. $\Delta U = 0$. - **Quasi-static Process:** Infinitely slow process, system is always in equilibrium. ### Laws of Thermodynamics #### Zeroth Law of Thermodynamics - If two systems are each in thermal equilibrium with a third system, they are also in thermal equilibrium with each other. - It defines temperature. #### First Law of Thermodynamics - **Statement:** Energy can neither be created nor destroyed; it can only be transformed from one form to another. - **Mathematical Form:** $\Delta Q = \Delta U + \Delta W$ - $\Delta Q$: Heat supplied to the system. - $\Delta U$: Change in internal energy of the system. - $\Delta W$: Work done by the system. - **Sign Conventions:** - $\Delta Q$ is positive if heat is added to the system. - $\Delta Q$ is negative if heat is removed from the system. - $\Delta W$ is positive if work is done by the system. - $\Delta W$ is negative if work is done on the system. - $\Delta U$ is positive if internal energy increases (temperature rises). - $\Delta U$ is negative if internal energy decreases (temperature falls). #### Work Done by a Gas - **General:** $\Delta W = \int P dV$ - **Isobaric:** $\Delta W = P(V_f - V_i)$ - **Isothermal:** $\Delta W = nRT \ln\left(\frac{V_f}{V_i}\right) = nRT \ln\left(\frac{P_i}{P_f}\right)$ - **Adiabatic:** $\Delta W = \frac{nR(T_i - T_f)}{\gamma - 1} = \frac{P_iV_i - P_fV_f}{\gamma - 1}$ - **Isochoric:** $\Delta W = 0$ #### Internal Energy ($\Delta U$) - For an ideal gas, internal energy depends only on temperature. - $\Delta U = n C_v \Delta T$ - $C_v$: Molar specific heat at constant volume. - **Degrees of Freedom (f):** - Monatomic (He, Ne, Ar): f = 3 (translational) - Diatomic (O$_2$, N$_2$): f = 5 (3 translational + 2 rotational) - Polyatomic (CO$_2$, NH$_3$): f = 6 (3 translational + 3 rotational) - **Relation between $C_v$ and f:** $C_v = \frac{f}{2}R$ - **Hence:** $\Delta U = n \frac{f}{2} R \Delta T$ #### Second Law of Thermodynamics - **Clausius Statement:** Heat cannot flow spontaneously from a colder body to a hotter body without external work. - **Kelvin-Planck Statement:** It is impossible to construct a device which operates in a cycle and produces no other effect than the extraction of heat from a single reservoir and the performance of an equivalent amount of work. - **Entropy (S):** A measure of disorder or randomness in a system. - $\Delta S = \frac{\Delta Q}{T}$ (for a reversible process) - In an isolated system, entropy never decreases ($\Delta S_{total} \ge 0$). #### Third Law of Thermodynamics - The entropy of a perfect crystalline solid at absolute zero temperature (0 K) is zero. ### Specific Heats and Relations #### Molar Specific Heats - **At Constant Volume ($C_v$):** Heat required to raise temperature of 1 mole of gas by 1 K at constant volume. - **At Constant Pressure ($C_p$):** Heat required to raise temperature of 1 mole of gas by 1 K at constant pressure. - **Relation between $C_p$ and $C_v$ (Mayer's Formula):** $C_p - C_v = R$ (where R is universal gas constant) - **Ratio of Specific Heats ($\gamma$):** $\gamma = \frac{C_p}{C_v} = 1 + \frac{2}{f}$ - Monatomic: $\gamma = 1 + \frac{2}{3} = \frac{5}{3} \approx 1.67$ - Diatomic: $\gamma = 1 + \frac{2}{5} = \frac{7}{5} = 1.4$ - Polyatomic: $\gamma = 1 + \frac{2}{6} = \frac{4}{3} \approx 1.33$ #### Heat Capacity - **Heat Capacity (C):** Amount of heat required to raise the temperature of a substance by $1^\circ$C or 1 K. $C = \frac{\Delta Q}{\Delta T}$. - **Specific Heat Capacity (c):** Heat capacity per unit mass. $c = \frac{C}{m}$. - $\Delta Q = mc \Delta T$ ### Heat Engines & Refrigerators #### Heat Engine - A device that converts heat energy into mechanical work. - **Working:** Takes heat ($Q_H$) from a hot reservoir ($T_H$), converts part of it into work ($W$), and rejects the remaining heat ($Q_C$) to a cold reservoir ($T_C$). - **Efficiency ($\eta$):** $\eta = \frac{\text{Work Done}}{\text{Heat Input}} = \frac{W}{Q_H} = \frac{Q_H - Q_C}{Q_H} = 1 - \frac{Q_C}{Q_H}$ - **Carnot Engine (Ideal Engine):** Most efficient engine operating between two temperatures. - $\eta_{Carnot} = 1 - \frac{T_C}{T_H}$ (Temperatures must be in Kelvin) - Carnot cycle consists of two isothermal and two adiabatic processes. #### Refrigerator / Heat Pump - A device that transfers heat from a cold reservoir to a hot reservoir, requiring external work. - **Working:** Extracts heat ($Q_C$) from a cold reservoir ($T_C$), external work ($W$) is done on it, and it rejects heat ($Q_H$) to a hot reservoir ($T_H$). - **Coefficient of Performance (COP):** - **Refrigerator:** $\text{COP}_{ref} = \frac{\text{Heat extracted from cold reservoir}}{\text{Work Done}} = \frac{Q_C}{W} = \frac{Q_C}{Q_H - Q_C}$ - **Heat Pump:** $\text{COP}_{HP} = \frac{\text{Heat delivered to hot reservoir}}{\text{Work Done}} = \frac{Q_H}{W} = \frac{Q_H}{Q_H - Q_C}$ - **For Carnot Refrigerator/Heat Pump:** - $\text{COP}_{ref, Carnot} = \frac{T_C}{T_H - T_C}$ - $\text{COP}_{HP, Carnot} = \frac{T_H}{T_H - T_C}$ - **Relation:** $\text{COP}_{HP} = \text{COP}_{ref} + 1$ ### Important Formulas & Relations - **Ideal Gas Equation:** $PV = nRT$ or $PV = Nk_B T$ - $n$: number of moles - $R$: Universal Gas Constant (8.314 J/mol·K) - $N$: number of molecules - $k_B$: Boltzmann Constant ($1.38 \times 10^{-23}$ J/K) - **Adiabatic Relations:** - $P_1V_1^\gamma = P_2V_2^\gamma$ - $T_1V_1^{\gamma-1} = T_2V_2^{\gamma-1}$ - $T_1P_1^{\frac{1-\gamma}{\gamma}} = T_2P_2^{\frac{1-\gamma}{\gamma}}$ or $T_1^{\gamma}P_1^{1-\gamma} = T_2^{\gamma}P_2^{1-\gamma}$ - **Mean Free Path ($\lambda$):** Average distance covered by a molecule between two successive collisions. - $\lambda = \frac{1}{\sqrt{2} \pi d^2 n_v}$ (where $d$ is molecular diameter, $n_v$ is number density) ### Kinetic Theory of Gases (Basics) - **Assumptions:** - Gas consists of a large number of identical, tiny, rigid, elastic spheres (molecules). - Molecules are in constant, random motion. - Collisions between molecules and with walls are perfectly elastic. - Volume of molecules is negligible compared to gas volume. - No intermolecular forces except during collisions. - **Pressure Exerted by Gas:** $P = \frac{1}{3} \frac{mN}{V} v_{rms}^2 = \frac{1}{3} \rho v_{rms}^2$ - **Average Kinetic Energy per Molecule:** $E_{avg} = \frac{3}{2} k_B T$ - **Total Internal Energy of Ideal Gas:** $U = \frac{f}{2} nRT$ - **Root Mean Square Speed ($v_{rms}$):** $v_{rms} = \sqrt{\frac{3RT}{M}} = \sqrt{\frac{3k_B T}{m}}$ - $M$: Molar mass - $m$: Mass of one molecule - **Average Speed ($v_{avg}$):** $v_{avg} = \sqrt{\frac{8RT}{\pi M}}$ - **Most Probable Speed ($v_{mp}$):** $v_{mp} = \sqrt{\frac{2RT}{M}}$ - **Relation:** $v_{mp} ### Calorimetry - **Principle of Calorimetry:** Heat lost by hot body = Heat gained by cold body. - **Latent Heat (L):** Heat absorbed or released per unit mass during a phase change at constant temperature. - $Q = mL$ - **Latent Heat of Fusion ($L_f$):** For melting/freezing. - **Latent Heat of Vaporization ($L_v$):** For boiling/condensation.