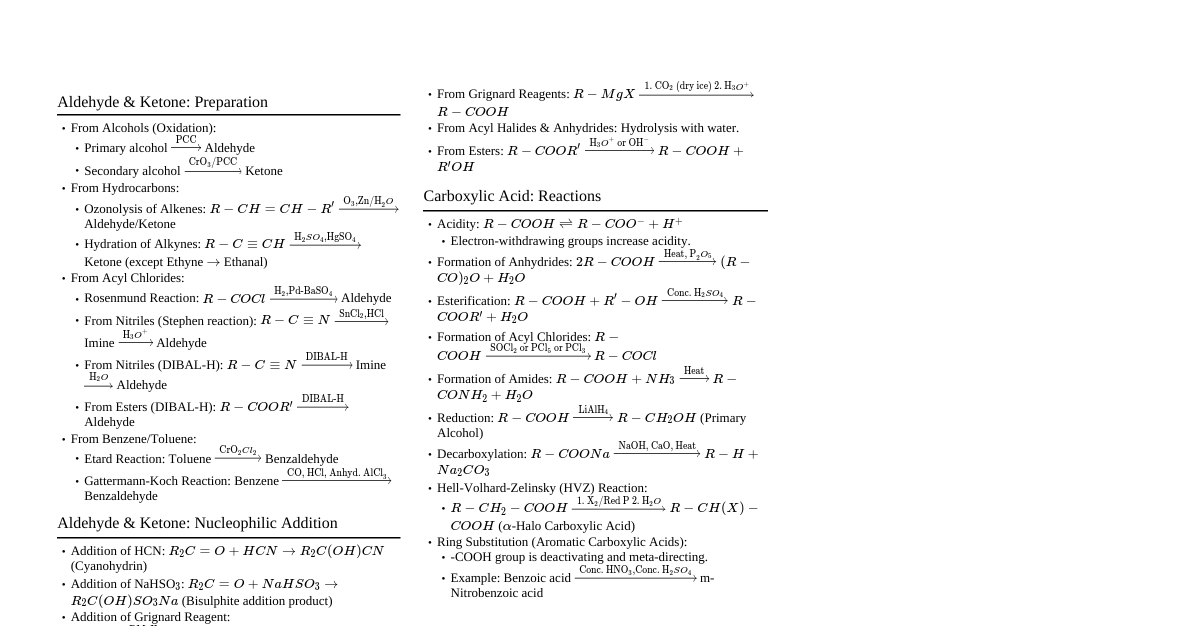
Aldehydes, Ketones and Carboxylic Acids Carbonyl compounds ($>C=O$) are crucial in organic chemistry. Aldehydes have carbonyl group bonded to carbon and hydrogen, ketones to two carbons. Carboxylic acids have carbonyl group bonded to carbon/hydrogen and a hydroxyl moiety (-OH). General Formulas Aldehyde: R-CHO Ketone: R-CO-R' Carboxylic Acid: R-COOH Acyl Halide: R-COX (X = Halogen) Amide: R-CONH$_2$ Ester: R-COOR' Acid Anhydride: R-CO-O-CO-R' Nomenclature of Aldehydes and Ketones Common Names Aldehydes: Derived from corresponding carboxylic acids by replacing '-ic acid' with '-aldehyde'. Substituent positions indicated by Greek letters ($\alpha, \beta, \gamma$, etc., starting from carbon next to aldehyde group). Acetaldehyde: $\text{CH}_3\text{CHO}$ Benzaldehyde: $\text{C}_6\text{H}_5\text{CHO}$ $\beta$-Bromobutyraldehyde: $\text{BrCH}_2\text{CH}_2\text{CH}_2\text{CHO}$ (incorrect structure in original, corrected to $\text{CH}_3\text{CH(Br)CH}_2\text{CHO}$) Ketones: Named by listing alkyl/aryl groups bonded to carbonyl, followed by 'ketone'. Acetone (dimethyl ketone): $\text{CH}_3\text{COCH}_3$ Acetophenone (methyl phenyl ketone): $\text{C}_6\text{H}_5\text{COCH}_3$ Propiophenone (ethyl phenyl ketone): $\text{C}_6\text{H}_5\text{COCH}_2\text{CH}_3$ Benzophenone (diphenyl ketone): $\text{C}_6\text{H}_5\text{COC}_6\text{H}_5$ IUPAC Names Aldehydes: Alkane name, replace '-e' with '-al'. Longest chain numbered from aldehyde carbon. If aldehyde group is on a ring, suffix '-carbaldehyde' is used. Ketones: Alkane name, replace '-e' with '-one'. Numbering starts from end nearer to carbonyl group. Cyclic ketones: carbonyl carbon is 1. Examples of Common and IUPAC Names (Table 8.1) Structure Common Name IUPAC Name $\text{HCHO}$ Formaldehyde Methanal $\text{CH}_3\text{CHO}$ Acetaldehyde Ethanal $(\text{CH}_3)_2\text{CHCHO}$ Isobutyraldehyde 2-Methylpropanal $\text{CH}_3\text{COCH}_3$ Dimethyl ketone (Acetone) Propan-2-one $\text{CH}_3\text{CH}_2\text{COCH}_2\text{CH}_3$ Diethyl ketone Pentan-3-one Structure of the Carbonyl Group Carbonyl carbon is $sp^2$-hybridised, forming three $\sigma$ bonds. Fourth valence electron forms a $\pi$-bond with oxygen. Carbonyl carbon and three attached atoms are coplanar, with bond angles approximately $120^\circ$. Carbon-oxygen double bond is polarised ($C^{\delta+}=O^{\delta-}$). Carbonyl carbon is electrophilic, carbonyl oxygen is nucleophilic. High polarity due to resonance: $R_2C=O \leftrightarrow R_2C^+-O^-$ Preparation of Aldehydes and Ketones 1. From Alcohols Oxidation: Primary alcohols $\xrightarrow{\text{Oxidation}}$ Aldehydes; Secondary alcohols $\xrightarrow{\text{Oxidation}}$ Ketones. Dehydrogenation: Alcohol vapours over heavy metal catalysts (Ag or Cu). Primary alcohols $\xrightarrow{\text{Cu/Ag, Heat}}$ Aldehydes; Secondary alcohols $\xrightarrow{\text{Cu/Ag, Heat}}$ Ketones. 2. From Hydrocarbons Ozonolysis of Alkenes: Alkenes $\xrightarrow{\text{1. O}_3 \text{ 2. Zn/H}_2\text{O}}$ Aldehydes/Ketones. Hydration of Alkynes: Ethyne $\xrightarrow{\text{H}_2\text{SO}_4/\text{HgSO}_4}$ Acetaldehyde. Other alkynes $\xrightarrow{\text{H}_2\text{SO}_4/\text{HgSO}_4}$ Ketones. 3. Preparation of Aldehydes From Acyl Chlorides (Rosenmund Reduction): $\text{RCOCl} \xrightarrow{\text{H}_2/\text{Pd-BaSO}_4}$ $\text{RCHO}$. From Nitriles and Esters: Stephen Reaction: $\text{RCN} \xrightarrow{\text{1. SnCl}_2/\text{HCl 2. H}_2\text{O}}$ $\text{RCHO}$. Using DIBAL-H: $\text{RCN} \xrightarrow{\text{1. DIBAL-H 2. H}_2\text{O}}$ $\text{RCHO}$; $\text{RCOOR}' \xrightarrow{\text{1. DIBAL-H 2. H}_2\text{O}}$ $\text{RCHO}$. From Hydrocarbons (Aromatic Aldehydes): Oxidation of Methylbenzene: Using Chromyl Chloride (Etard Reaction): $\text{Toluene} \xrightarrow{\text{1. CrO}_2\text{Cl}_2/\text{CS}_2 \text{ 2. H}_3\text{O}^+}$ Benzaldehyde. Using Chromic Oxide: $\text{Toluene} \xrightarrow{\text{1. CrO}_3/(\text{CH}_3\text{CO})_2\text{O} \text{ 2. H}_3\text{O}^+}$ Benzaldehyde. Side Chain Chlorination: $\text{Toluene} \xrightarrow{\text{Cl}_2/\text{hv}} \text{Benzal chloride} \xrightarrow{\text{H}_2\text{O}/\text{Heat}}$ Benzaldehyde. Gatterman-Koch Reaction: $\text{Benzene} \xrightarrow{\text{CO, HCl}/\text{Anhyd. AlCl}_3/\text{CuCl}}$ Benzaldehyde. 4. Preparation of Ketones From Acyl Chlorides: $\text{2R'COCl} + \text{R}_2\text{Cd} \rightarrow \text{2R'COR} + \text{CdCl}_2$. ($\text{R}_2\text{Cd}$ from $\text{2RMgX} + \text{CdCl}_2$) From Nitriles: $\text{RCN} \xrightarrow{\text{1. R'MgX 2. H}_2\text{O}}$ $\text{RCOR}'$. From Benzene (Friedel-Crafts Acylation): $\text{Benzene} + \text{RCOCl} \xrightarrow{\text{Anhyd. AlCl}_3}$ $\text{C}_6\text{H}_5\text{COR}$. Physical Properties of Aldehydes and Ketones Methanal is a gas, ethanal is a volatile liquid. Others are liquids or solids. Higher boiling points than hydrocarbons/ethers of comparable molecular mass due to dipole-dipole interactions. Lower boiling points than alcohols of comparable molecular mass due to absence of intermolecular H-bonding. Lower members (methanal, ethanal, propanone) are miscible with water due to H-bonding. Solubility decreases with increasing alkyl chain length. Lower aldehydes have sharp pungent odours; larger molecules are less pungent, more fragrant. Chemical Reactions of Aldehydes and Ketones 1. Nucleophilic Addition Reactions Aldehydes and ketones undergo nucleophilic addition. Mechanism: Nucleophile attacks electrophilic carbonyl carbon (from $sp^2$ to $sp^3$ hybridisation). Tetrahedral alkoxide intermediate forms, which captures a proton. Reactivity: Aldehydes are generally more reactive than ketones due to: Steric Reasons: Less steric hindrance around carbonyl carbon in aldehydes. Electronic Reasons: Alkyl groups reduce electrophilicity of carbonyl carbon; ketones have two alkyl groups. Benzaldehyde is less reactive than propanal due to resonance stabilization reducing electrophilicity of carbonyl carbon. Examples: Addition of HCN: $\text{RCHO} + \text{HCN} \rightarrow \text{RCH(OH)CN}$ (Cyanohydrin). Catalysed by base. Addition of $\text{NaHSO}_3$: Forms crystalline bisulphite addition product, useful for separation/purification. Addition of Alcohols: Aldehydes + 1 eq. alcohol $\xrightarrow{\text{HCl gas}}$ Hemiacetal. Hemiacetal + 1 eq. alcohol $\xrightarrow{\text{HCl gas}}$ Acetal. Ketones + Ethylene glycol $\xrightarrow{\text{HCl gas}}$ Ethylene glycol ketal (cyclic product). Addition of Ammonia Derivatives (H$_2$N-Z): Forms imines, oximes, hydrazones, semicarbazones etc. ($>C=O + H_2N-Z \rightarrow >C=N-Z + H_2O$) 2. Reduction To Alcohols: Aldehydes $\xrightarrow{\text{NaBH}_4 \text{ or LiAlH}_4}$ Primary alcohols; Ketones $\xrightarrow{\text{NaBH}_4 \text{ or LiAlH}_4}$ Secondary alcohols. To Hydrocarbons: Clemmensen Reduction: $\text{R}_2\text{CO} \xrightarrow{\text{Zn-Hg/HCl}}$ $\text{R}_2\text{CH}_2$. Wolff-Kishner Reduction: $\text{R}_2\text{CO} \xrightarrow{\text{1. NH}_2\text{NH}_2 \text{ 2. KOH/Ethylene glycol, Heat}}$ $\text{R}_2\text{CH}_2$. 3. Oxidation Aldehydes oxidise easily to carboxylic acids. Ketones oxidise under vigorous conditions with C-C bond cleavage. Tollens' Test (for Aldehydes): $\text{RCHO} + \text{2[Ag(NH}_3)_2]^+ \text{ + 3OH}^- \rightarrow \text{RCOO}^- + \text{2Ag(s)} + \text{2H}_2\text{O} + \text{4NH}_3$. (Silver mirror forms) Fehling's Test (for Aldehydes): $\text{RCHO} + \text{2Cu}^{2+} + \text{5OH}^- \rightarrow \text{RCOO}^- + \text{Cu}_2\text{O(s)} + \text{3H}_2\text{O}$. (Red-brown precipitate forms) Oxidation of Methyl Ketones (Haloform Reaction): Methyl ketones ($\text{RCOCH}_3$) $\xrightarrow{\text{NaOX}}$ Sodium carboxylate + Haloform ($\text{CHX}_3$). 4. Reactions due to $\alpha$-hydrogen $\alpha$-hydrogens of aldehydes/ketones are acidic due to electron-withdrawing carbonyl group and resonance stabilisation of conjugate base. Aldol Condensation: Aldehydes/ketones with $\alpha$-hydrogen $\xrightarrow{\text{Dilute alkali}}$ $\beta$-hydroxy aldehydes (aldols) or $\beta$-hydroxy ketones (ketols), which then dehydrate to $\alpha,\beta$-unsaturated carbonyl compounds. Cross Aldol Condensation: Between two different aldehydes/ketones. 5. Other Reactions Cannizzaro Reaction: Aldehydes without $\alpha$-hydrogen $\xrightarrow{\text{Conc. alkali}}$ Alcohol + Carboxylic acid salt (self-oxidation and reduction). E.g., Formaldehyde, Benzaldehyde. Electrophilic Substitution: Aromatic aldehydes/ketones undergo meta-directing substitution. Nomenclature of Carboxylic Acids Common Names Derived from Latin/Greek names of their natural sources (e.g., Formic acid from ants). End with '-ic acid'. IUPAC Names Alkane name, replace '-e' with '-oic acid'. Carboxyl carbon is numbered 1. For compounds with multiple carboxyl groups, use prefixes like 'dicarboxylic acid'. Examples of Common and IUPAC Names (Table 8.3) Structure Common Name IUPAC Name $\text{HCOOH}$ Formic acid Methanoic acid $\text{CH}_3\text{COOH}$ Acetic acid Ethanoic acid $\text{CH}_3\text{CH}_2\text{COOH}$ Propionic acid Propanoic acid $\text{HOOC-COOH}$ Oxalic acid Ethanedioic acid $\text{HOOC-(CH}_2)_4\text{-COOH}$ Adipic acid Hexanedioic acid Structure of Carboxyl Group Bonds to carboxyl carbon are coplanar, separated by $\approx 120^\circ$. Carboxyl carbon is less electrophilic than carbonyl carbon due to resonance: $\text{R-C(=O)-OH} \leftrightarrow \text{R-C(O}^-\text{)=OH}^+$ Preparation of Carboxylic Acids 1. From Primary Alcohols and Aldehydes Primary alcohols $\xrightarrow{\text{Oxidising agents (KMnO}_4, \text{K}_2\text{Cr}_2\text{O}_7, \text{CrO}_3\text{)}}$ Carboxylic acids. Aldehydes $\xrightarrow{\text{Mild oxidising agents}}$ Carboxylic acids. 2. From Alkylbenzenes Alkylbenzenes $\xrightarrow{\text{Vigorous oxidation (KMnO}_4 \text{ or Chromic Acid)}}$ Aromatic carboxylic acids. 3. From Nitriles and Amides Nitriles $\xrightarrow{\text{H}^+ \text{ or OH}^- \text{ catalyst}}$ Amides $\xrightarrow{\text{H}^+ \text{ or OH}^- \text{ catalyst}}$ Carboxylic acids. 4. From Grignard Reagents $\text{RMgX} + \text{CO}_2 \xrightarrow{\text{Dry ether}}$ $\text{RCOOMgX} \xrightarrow{\text{H}_3\text{O}^+}$ $\text{RCOOH}$. 5. From Acyl Halides and Anhydrides Acyl halides $\xrightarrow{\text{H}_2\text{O}}$ Carboxylic acids. Anhydrides $\xrightarrow{\text{H}_2\text{O}}$ Carboxylic acids. 6. From Esters Esters $\xrightarrow{\text{H}^+ \text{ or OH}^-, \text{H}_2\text{O}}$ Carboxylic acids. Physical Properties of Carboxylic Acids Lower members are colourless liquids with unpleasant odours; higher acids are waxy solids, practically odourless. Higher boiling points than aldehydes, ketones, and alcohols due to extensive intermolecular H-bonding (exist as dimers). Lower members are miscible with water due to H-bonding. Solubility decreases with increasing carbon chain. Chemical Reactions of Carboxylic Acids 1. Reactions Involving Cleavage of O-H Bond (Acidity) Carboxylic acids are acidic, reacting with metals and alkalies to form salts. Stronger acids than alcohols and phenols. Resonance stabilisation of carboxylate ion $\text{RCOO}^-$ makes them acidic: $\text{R-C(=O)-O}^- \leftrightarrow \text{R-C(O}^-\text{)=O}$. Effect of Substituents on Acidity: Electron-withdrawing groups (EWG) increase acidity by stabilising carboxylate ion. Electron-donating groups (EDG) decrease acidity by destabilising carboxylate ion. 2. Reactions Involving Cleavage of C-OH Bond Formation of Anhydride: Carboxylic acids $\xrightarrow{\text{Heat, H}_2\text{SO}_4 \text{ or P}_2\text{O}_5}$ Anhydrides. Esterification: $\text{RCOOH} + \text{R'OH} \xrightarrow{\text{H}^+}$ $\text{RCOOR}' + \text{H}_2\text{O}$. Reactions with $\text{PCl}_5, \text{PCl}_3, \text{SOCl}_2$: $\text{RCOOH} \xrightarrow{\text{PCl}_5 \text{ or PCl}_3 \text{ or SOCl}_2}$ $\text{RCOCl}$. Reaction with Ammonia: $\text{RCOOH} + \text{NH}_3 \rightarrow \text{RCOONH}_4 \xrightarrow{\text{Heat}}$ $\text{RCONH}_2$. 3. Reactions Involving -COOH Group Reduction: $\text{RCOOH} \xrightarrow{\text{1. LiAlH}_4 \text{ or B}_2\text{H}_6 \text{ 2. H}_3\text{O}^+}$ $\text{RCH}_2\text{OH}$. Decarboxylation: $\text{RCOONa} \xrightarrow{\text{NaOH/CaO, Heat}}$ $\text{RH} + \text{Na}_2\text{CO}_3$. 4. Substitution Reactions in the Hydrocarbon Part Halogenation (Hell-Volhard-Zelinsky Reaction): $\text{RCH}_2\text{COOH} \xrightarrow{\text{1. X}_2/\text{Red P 2. H}_2\text{O}}$ $\text{RCHXCOOH}$. Ring Substitution: Aromatic carboxylic acids undergo meta-directing electrophilic substitution. (Do not undergo Friedel-Crafts reaction).