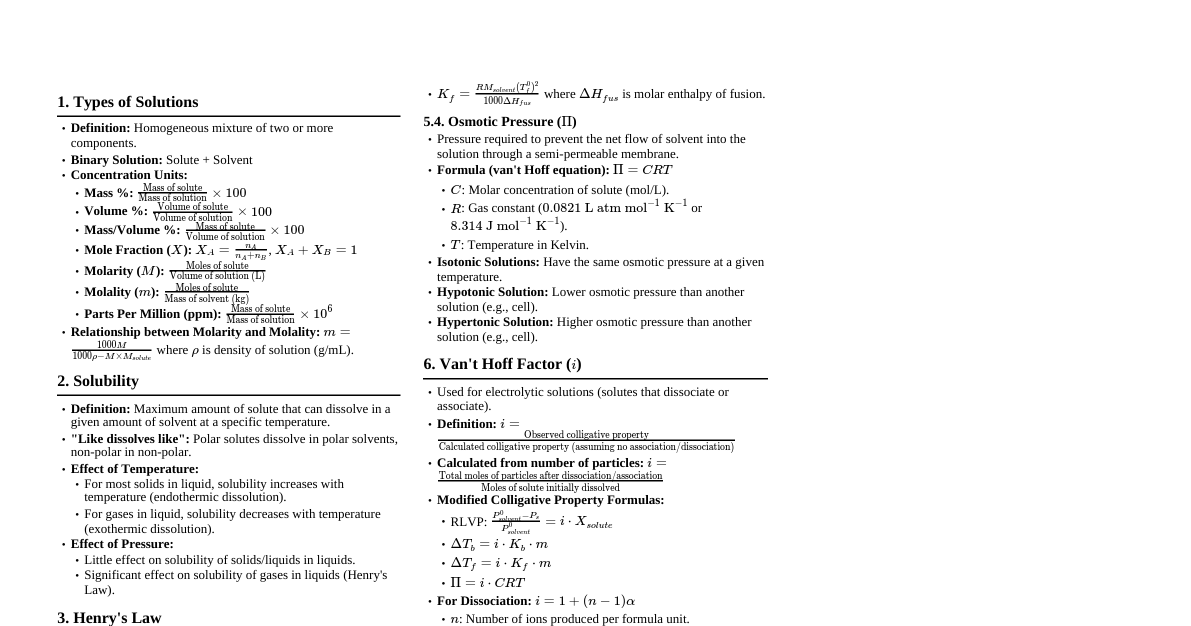
Water: The Essence of Life Colorless, transparent, odourless liquid. Exists in three states: solid (ice), liquid (water), gas (vapour). Covers 71% of Earth's surface. Basic for all life forms. Importance of Water for Humans Human adult body is 60-65% water. A 2% decrease in body water can cause fatigue; 10% decrease can be fatal. Average adult male needs 2.5-3.7 liters per day. Average adult female needs 2-2.7 liters per day. Regulates body temperature through sweating. Transports nutrients and facilitates chemical reactions. Aids excretion of toxic nitrogenous waste (urea). Water and Civilizations Shaped human societies, settling near water sources. Examples: Egyptian civilization on the Nile. Chinese civilization on Huang He and Yangtze. Indus Valley Civilization (present Pakistan). Water and Religion Considered 'sacred' in major religions (Christianity, Islam, Judaism, Hinduism, Buddhism). Unique properties and therapeutic effects lead to its role in religious rituals. Occurrence of Water Covers nearly $3/4^{th}$ of Earth's surface. 97% is saltwater (oceans). Only 3% is freshwater. 68% of freshwater is trapped in glaciers and polar ice caps. Very small amount accessible in groundwater, lakes, streams, rivers. Consumption by Living Organisms Plants use water for photosynthesis. Water dissolves food produced by plants (sucrose) for transport. Water helps plants maintain turgid and parallel to the ground. Water allows aquatic plants to carry out photosynthesis. Fishes use dissolved oxygen in water (via gills). Water Cycle (Hydrological Cycle) Evaporation Water evaporates from lakes, rivers, oceans, rainfall, etc. Water vapour forms in the air. Transpiration: process by which plants release water from soil in the form of water vapour. Animals and humans release water from their bodies as sweat and excretion. Solar radiation is the major source of energy for this process. Condensation Water vapour transforms into water droplets in the air, creating clouds, fog, etc. Precipitation Condensed water vapour falls to Earth's surface as rain, snow, mist, etc. Infiltration and Percolation Infiltration: flow of water from ground surface into the ground. Percolation: water moving downwards through soil and rocks under gravity. Surface Runoff and Collection Water moves across land as melting ice/snow, rain. Infiltrates into the ground, evaporates into the air, or stored in lakes/reservoirs. Subsurface Flow Movement of water underground (aquifers). Can resurface through springs or be extracted via wells. Sources of Water Only 3% of Earth's water is freshwater. Approximately 1.2% can be used for various activities. Freshwater is maintained by precipitation. Distributed as lakes, streams, rivers, ponds. Groundwater: Water held underground in soil pores and rock crevices. Aquifer: body of permeable rock storing groundwater. Groundwater is critical, making up 30% of total freshwater. Safeguarding groundwater prevents water shortage. Relatively less polluted, main choice for drinking water. Nitrates from agricultural activities are common groundwater contaminants. Water - The Universal Solvent Dissolves a large variety of chemical substances. Homogeneous mixture: uniform composition throughout. Solution: homogeneous mixture of two or more liquids. Solute: substance dissolved in a solvent. Solvent: substance that dissolves a solute. Aqueous solution: a solution where water is the solvent. Solubility and Saturated Solutions Solubility: maximum quantity of solute that can dissolve in a certain amount of solvent at a specific temperature. Unsaturated solution: more solute can be dissolved at a particular temperature. Saturated solution: no more solute can be dissolved at a particular temperature. Example: Solubility of sodium chloride is 36 g per 100 ml of water at $20^\circ C$. Factors Affecting Solubility Nature of the solute and solvent: "Like dissolves like". Example: Sodium chloride (ionic) dissolves well in water (polar). Sugar (polar) also dissolves well. Sugar has higher solubility (204 g/100 ml water at $20^\circ C$) than sodium chloride. Temperature: For most solids, solubility increases with temperature. Example: Solubility of sodium chloride increases from 36 g to 39 g per 100 ml of water from $20^\circ C$ to $100^\circ C$. For gaseous solutes, solubility decreases with increasing temperature (e.g., carbonated drinks). Pressure: Little effect on solubility of solid/liquid solutes. For gaseous solutes, solubility increases with pressure. Example: Opening a carbonated drink bottle reduces pressure, releasing dissolved gas. Forces Exhibited by Water Cohesive and Adhesive Forces Cohesive forces: forces that act between the molecules of the same substance. Creates a "skin" on the surface, making it resistant to separation. Example: Water molecules are pulled together tightly, forming droplets. Even rainwater falls in the form of droplets rather than a mist due to these strong cohesive forces. Adhesive forces: forces that act between molecules of different substances. Example: Water sticking to surfaces (e.g., car mirrors). When a thin straw is placed in a glass of water, the water is pulled up into the straw to a greater height than the level of water in the glass. This phenomenon is known as capillary action , which results from the cohesive forces between water molecules and the adhesive forces between the straw and water molecules. Capillary action occurs when the adhesive forces between the water and the straw are stronger than the cohesive forces between the water. Plants draw water, along with the dissolved minerals, from the roots to the tip via thin narrow xylem vessels using capillary action. Continuous random movement of particles suspended by liquids or gases resulting from the collisions of molecules of the surrounding medium is termed Brownian motion. Potable Water According to the World Health Organisation (WHO), between 50 and 100 litres of water per person per day is needed to ensure that most basic needs are met per day for drinking, food preparation for drinking and cooking water, which is safe for human consumption, is called potable water . Wells, springs, lakes, streams, ponds, and rivers are generally considered unsafe for human consumption (non-potable) as they may contain unknown harmful impurities, toxic contaminants, bacteria and viruses. Lack of sanitary facilities and improper disposal of human waste, along with poor sewage systems, result in contaminating water with pathogens that can cause serious waterborne diseases such as diarrhoea, typhoid, cholera and more. Characteristics of Potable Water It should be colourless and odourless. It should contain dissolved gases like oxygen and carbon dioxide. It should be free from pathogens, specifically the ones that affect the digestive tract of humans and animals. It should be free of toxic metals such as mercury and lead, radioactive substances, urea and harmful salts like cyanides. It should contain small amounts of essential mineral salts of calcium, magnesium and sodium, which give it a pleasant taste. Water pollution is caused by various sources, including farming practices that contaminate water with pesticides and fertilisers. Many industries also release their waste into water bodies which further pollutes the water with toxic chemicals such as asbestos, mercury, and lead. Thus, the water obtained from various sources has to be cleaned, filtered and treated by ultraviolet light, chlorination or by passing ozone. After testing the treated water to ensure that the water meets the local drinking water standards, it is termed fit for drinking or cooking purposes. Even after the treatment of water, it is critical that the distribution system that provides water to household functions optimally to maintain the characteristics of potable water. Jal Jeevan Mission is an initiative of the Indian government, the department of drinking water and sanitation. It aims to deliver clean and sufficient drinking water to all households in rural India by 2024. Aquatic animals are forced out from rivers and ponds due to the overflow of waterbodies. In the areas of low vegetation, it washes the top layer of the soil leading to soil erosion. Thus, floods and droughts can cause severe damage to life and property. Water Pollution Water pollution is defined as any change in the quality of water due to the addition of harmful substances that makes it unsuitable for use by humans and other living organisms. Agricultural Waste Fertilisers used in agriculture contain nitrates and phosphates, which are often washed off as runoff to nearby waterbodies. These added nutrients cause excessive growth of algae . The algae after death serve as food for bacteria that result in the depletion of oxygen from water, killing aquatic life. Oil Spills Oil spills are the accidental discharge of crude oil from tankers at sea, or due to disasters at oil platforms or leaks from underground storage tanks. Oil spills kill a large number of marine plants and wildlife. Since crude oil is lighter than water, it floats on the surface and blocks out light, preventing photosynthesis by aquatic plants. The oil suffocates fish by coating their gills. The oil causes wetting of bird feathers and prevents them from flying, reduces waterproofing and exposes them to extremes in temperature. Floods An area may experience a natural disaster known as a flood when it receives an unusually excessive amount of rainfall. When it rains heavily for several days such that it exceeds the holding capacity of the soil, then the water starts accumulating over the land. It is known as water logging . Eventually, this condition leads to rivers and ponds overflowing and land submerging. This situation is called a flood. Water logging and floods can displace air in the soil, affecting subterranean life, including earthworms and snakes. In addition, the absence of air and excess water can degrade the roots of plants, leading to their death and ruining crops. Droughts When an area experiences insufficient rainfall for consecutive seasons, coupled with abnormally dry weather, it can result in a prolonged drought . Drought can be characterised by dried soil with visibly large cracks in it. Drought can be characterised by dried soil with visibly large cracks in it. Drought-up rivers, lakes, ponds, etc. Due to scarcity of water, the land becomes infertile, eventually leading to shortage of food and fodder . It also adversely affected, which leads to a shortage of food and fodder. It also adversely affects animal life in many ways, one of which is the death of animals due to thirst and hunger. Thus, the loss of food and water leads to poor health conditions affecting the ecosystem. Droughts and Floods As much as water is essential to us, it can also be detrimental to life in both its absence and abundance. It can be used to increase the groundwater level in aquifers. It reduces the runoff of loss of rainfall and prevents flooding in urban areas. It provides increased water supply for agricultural purposes, thereby enhancing the crop yield. It provides water supply during the dry months of the year. Water Management World Water Day is celebrated each year on $22^{nd}$ of March to increase awareness among people about the importance, need and conservation of water. This day was formally proposed at the United Nations Conference on Environment and Development in Rio de Janeiro, Brazil in 1992 and the first World Water Day was celebrated by the United Nations General Assembly in 1993. Each year , an annual theme is selected by UN-Water and the day is celebrated to highlight the importance of water. Activities are organised, such as musical celebrations, educational events, excursions to local waterbodies and campaigns, to raise funds for access to clean water. In 2022, the theme was 'Groundwater - Making the Invisible Visible' and it was focused on the conservation of groundwater. Of the entire world's water supply, less than approximately 1% is available for human use. Considering that 100 litres are the total amount of water in the world, about half a tablespoon of it is available for consumption. Each year, the human population increases by around 75 million, putting immense pressure on the already dwindling supply of freshwater. The scarcity of water can be overcome by water management, which includes the conservation of water, minimising wastage and reusing wastewater. Water management helps in lowering water and sewer-related costs, better irrigation practices, especially during summers, saving energy and thus leading to environmental preservation. Water Consumption Can Be Drastically Reduced by Making Minor Lifestyle Changes Remember to turn off the tap while brushing your teeth. A running tap wastes 6 litres of water per minute. Use dual flush toilets which gives a choice of how much water to use. Use a low-flow showerhead as it reduces the amount of water used during a bath. Turn off the shower when soaping, then turn it back on for rinsing. A washing machine should be fully loaded for every single wash. Repair leaking pipelines or leaking water taps at the earliest. Use a wet cloth and a bucket of water to clean the car rather than using a hose pipe. Do not drain leftover drinking water in a glass or bottle, rather use it to water plants. Use treated wastewater for watering lawns, gardens, floors and even for construction purposes. Rainwater Harvesting Another promising alternative for increasing water supply is rainwater harvesting, i.e., collecting rainwater from rooftops or land surfaces and storing it for later use. This stored water can be used for gardening, irrigation, toilet flushing or washing activities. The basic components of any rainwater harvesting system can be categorised into four parts: A catchment area: to capture rainfall using either a rooftop or a land surface. A conveyance system: the use of gutter, drain spouts and pipes to carry the water from the roof to the storage system. A storage system: to hold the rainwater in a barrel or tank for future use. A distribution system: to transport water from storage tanks or barrels to where it is needed. Rainwater harvesting can provide the following benefits: Rainwater is low in minerals and provides high-quality soft water. There is less dependence on the government's main water supply, thereby reducing water bills. Potable water is water which is safe for human consumption for drinking and cooking purposes. Potable water is free from pathogens, toxic metals, harmful salts, essential minerals such as calcium, magnesium and sodium, along with nutrients. Rainwater harvesting involves the collection of rainwater from rooftops or land surfaces and storage for further use in gardening, irrigation, toilet flushing or washing activities. Water pollution is caused due to discharge of sewage and industrial waste into waterbodies. Runoff from agricultural practices and oil spills also pollute water.