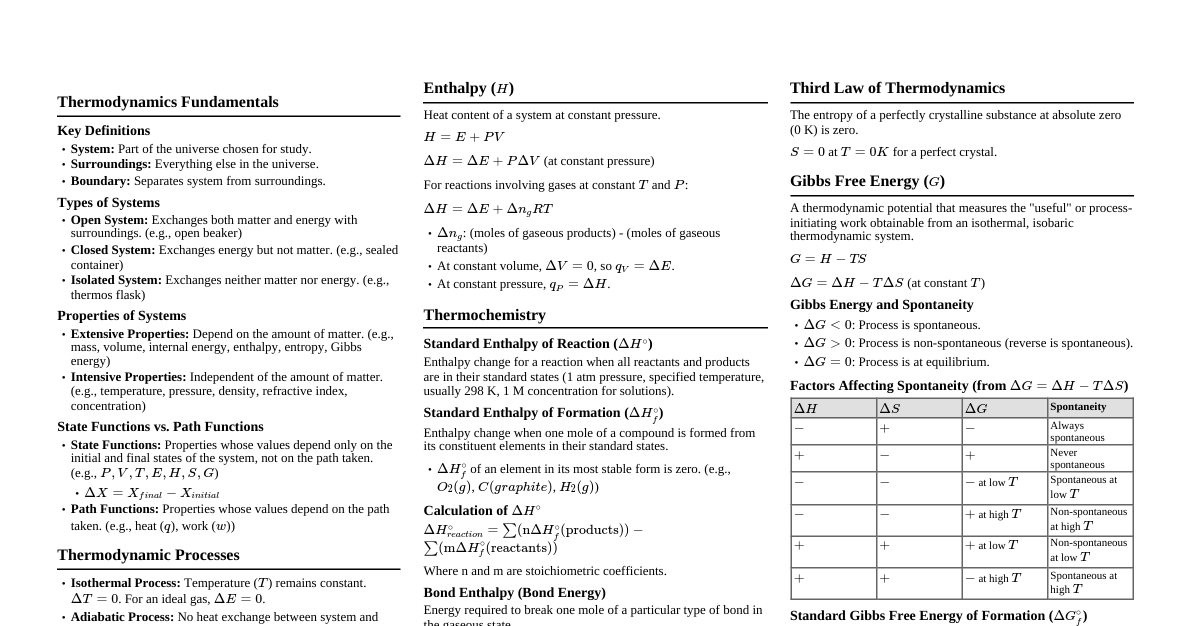
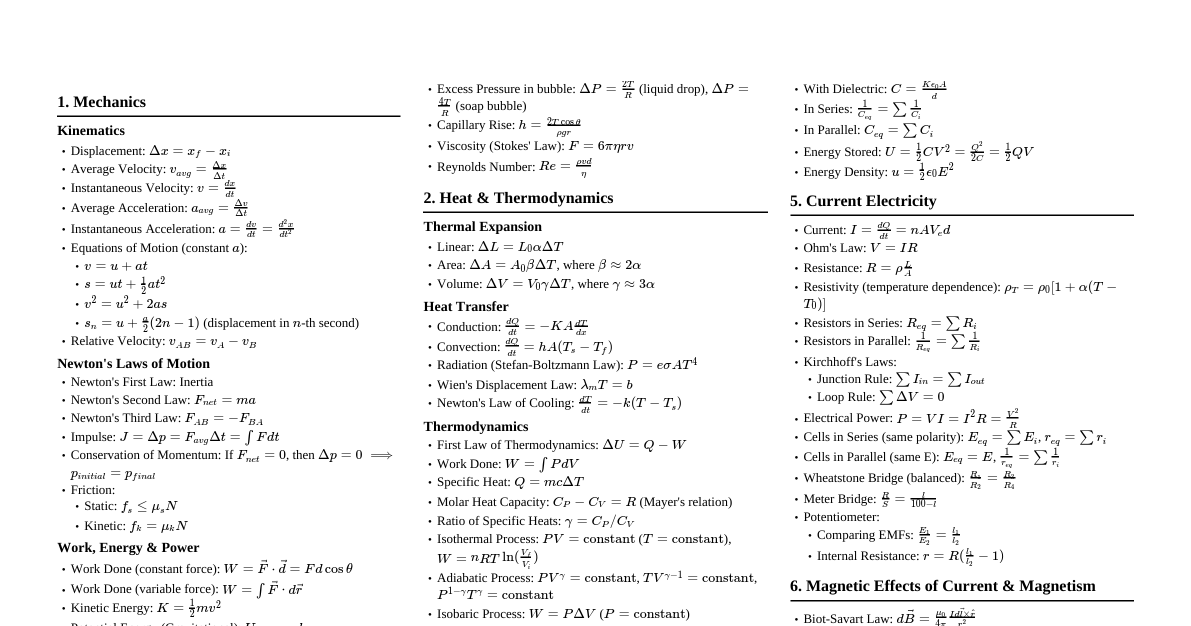
1. Introduction to the Second Law The First Law of Thermodynamics deals with the conservation of energy but doesn't indicate the direction of a process or the possibility of its occurrence. The Second Law establishes the criteria for spontaneity of a process and defines the concept of entropy. It states that processes occur in a certain direction and that energy has quality as well as quantity. 2. Statements of the Second Law 2.1. Kelvin-Planck Statement It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work. This implies that no heat engine can have a thermal efficiency of 100% or more. Some heat must be rejected to a low-temperature reservoir. A device violating this statement is called a Perpetual Motion Machine of the Second Kind (PMM2). 2.2. Clausius Statement It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher-temperature body. This means that heat cannot spontaneously flow from a colder body to a hotter body without external work input. Refrigerators and heat pumps require work input to achieve this transfer. 2.3. Equivalence of Statements The Kelvin-Planck and Clausius statements are equivalent. Violation of one implies violation of the other. 3. Reversible and Irreversible Processes Reversible Process: A process that can be reversed without leaving any trace on the surroundings. Both the system and surroundings return to their initial states. Requires infinitesimally small gradients for heat transfer, pressure differences, etc. Idealized processes, serve as theoretical limits for actual processes. Examples: Frictionless pendulum, slow expansion/compression. Irreversible Process: A process that cannot be reversed without leaving a change in the surroundings. All actual processes are irreversible. Due to factors like friction, unrestrained expansion, mixing, heat transfer across finite temperature difference, chemical reactions. These factors are called irreversibilities . 4. Carnot Cycle and Carnot Principles 4.1. Carnot Cycle (Reversible Cycle) Consists of four reversible processes: Isothermal heat addition ($Q_H$) at $T_H$. Reversible adiabatic expansion (work output). Isothermal heat rejection ($Q_L$) at $T_L$. Reversible adiabatic compression (work input). All heat engines operating on the Carnot cycle between the same two temperature reservoirs have the same efficiency. 4.2. Carnot Principles The efficiency of an irreversible heat engine is always less than the efficiency of a reversible one operating between the same two thermal reservoirs. The efficiencies of all reversible heat engines operating between the same two thermal reservoirs are the same. 4.3. Thermal Efficiency of Carnot Engine $\eta_{th,rev} = 1 - \frac{T_L}{T_H}$ Where $T_L$ and $T_H$ are absolute temperatures of the low and high temperature reservoirs, respectively. This is the maximum possible efficiency for any heat engine. 4.4. COP for Reversible Refrigerator/Heat Pump $COP_{R,rev} = \frac{T_L}{T_H - T_L}$ $COP_{HP,rev} = \frac{T_H}{T_H - T_L}$ 5. Entropy 5.1. Definition Entropy, $S$, is an extensive thermodynamic property of a system. It is a measure of the disorder or randomness of a system, or more fundamentally, a measure of the unavailability of a system's thermal energy for conversion into mechanical work. For a reversible process, the change in entropy is defined as: $dS = \left(\frac{\delta Q}{T}\right)_{rev}$ Units: J/K or kJ/K. 5.2. Clausius Inequality For any cycle, $\oint \frac{\delta Q}{T} \le 0$. For a reversible cycle: $\oint \frac{\delta Q}{T} = 0$. For an irreversible cycle: $\oint \frac{\delta Q}{T} This inequality provides the basis for defining entropy. 5.3. Principle of Increase of Entropy For an isolated system, the entropy always increases during an irreversible process and remains constant during a reversible process. It never decreases. $\Delta S_{isolated} \ge 0$ For a general process (system + surroundings): $\Delta S_{total} = \Delta S_{system} + \Delta S_{surroundings} \ge 0$ $\Delta S_{total} = 0$ for reversible processes. $\Delta S_{total} > 0$ for irreversible processes. 5.4. Entropy Change for Ideal Gases $s_2 - s_1 = c_v \ln\left(\frac{T_2}{T_1}\right) + R \ln\left(\frac{v_2}{v_1}\right)$ $s_2 - s_1 = c_p \ln\left(\frac{T_2}{T_1}\right) - R \ln\left(\frac{P_2}{P_1}\right)$ For constant specific heats. 5.5. Entropy Generation The increase in entropy of the universe is due to irreversibilities. $\Delta S_{total} = \int \frac{\delta Q}{T} + S_{gen}$ $S_{gen}$ is the entropy generated due to irreversibilities within the system boundary. $S_{gen} \ge 0$. 6. Third Law of Thermodynamics The entropy of a pure crystalline substance at absolute zero temperature is zero. This provides an absolute reference point for entropy. 7. Exergy (Availability) Exergy is the maximum useful work that can be obtained from a system at a specified state in a specified environment. It is a measure of the quality of energy. Exergy is consumed (destroyed) during an irreversible process. Exergy destruction: $X_{destroyed} = T_0 S_{gen}$, where $T_0$ is the environment temperature.