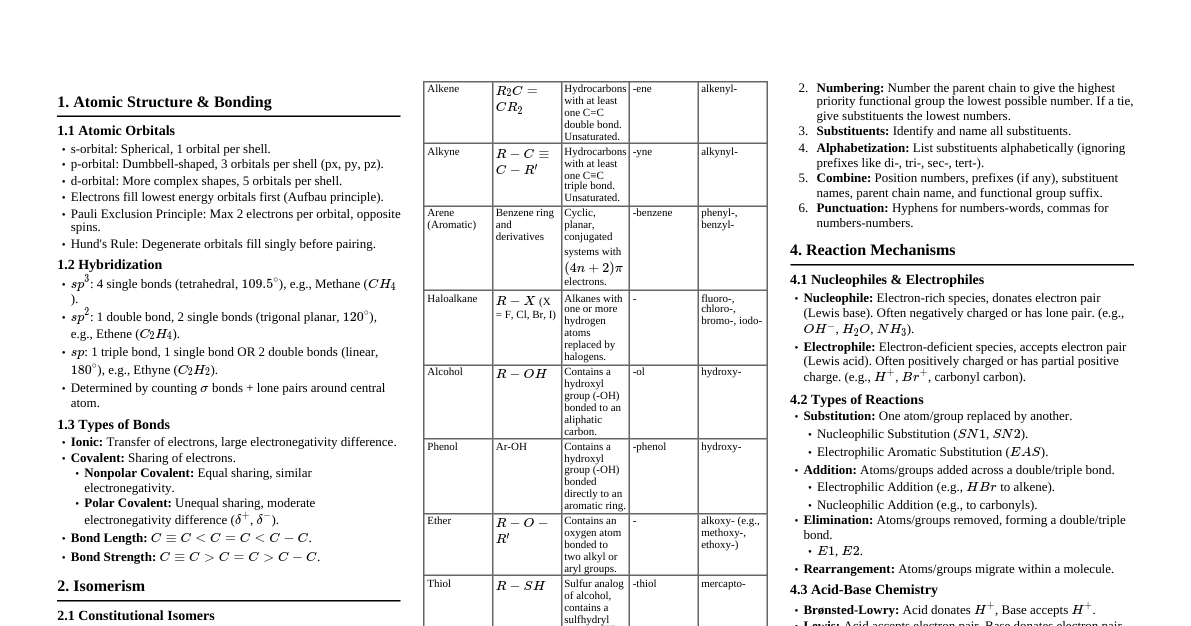
General Organic Chemistry Reaction: Breaking of old bonds and formation of new bonds. $$A-B + X-Y \rightarrow A-X + B-Y$$ Reaction Mechanism: Sequential account of electron movement, energetics, and kinetics. Substrate/Reactant: Species attacked by a reagent. Reagent: Species that attacks the substrate. Types of Bond Cleavage 1. Heterolytic Cleavage/Fission Unequal distribution of electrons, forming ions. Also known as ionic or heterolytic cleavage. Example: $R-X \rightarrow R^+ + X^-$ 2. Homolytic Cleavage/Fission Equal distribution of electrons, forming uncharged species (free radicals). Also known as unintonic or homolytic cleavage. Example: $R-X \rightarrow R^\cdot + X^\cdot$ Both cleavage types form reaction intermediates: positively charged, negatively charged, or neutral with unpaired electrons. Attacking Reagents Species that attack a substrate or intermediate to form a product. 1. Electrophilic Reagents (Electrophiles) Electron-loving species that attack the negative part of a molecule. Can be positively charged or electron-deficient molecules (sextet or septet). Positively charged electrophiles: $H^+, NO_2^+, R_3C^+$ Neutral electrophiles: Lewis acids: $BF_3, AlCl_3, SO_3$ Neutral atoms accepting electrons: $>C=O, R-Mg-X, R-X$ (the carbon atom in these can be electrophilic) Free radicals, carbenes, nitrenes are also electrophilic. 2. Nucleophilic Reagents (Nucleophiles) Nucleus-loving species that attack the positive site of a substrate. Can be negatively charged ions or possess a lone pair of electrons. Negatively charged nucleophiles: $OH^-, OR^-, CN^-, X^-(Halide), R-COO^-, SH^-$ Neutral nucleophiles (Lewis bases with lone pairs): $H_2O, R-OH, R-O-R, NH_3, R-NH_2, R_3N, R-SH, R-S-R, H_2S$ Ambident nucleophiles: Possess two electron-rich centers or atoms with unshared electron pairs. Examples: $NO_2^-, CN^-, SCN^-$ Amphiphile: Molecule with a multiple bond between carbon and a more electronegative atom, can act as both electrophile (at carbon) and nucleophile (at oxygen/nitrogen). For example, a carbonyl group $>C=O$. S.No. Electrophile Nucleophile 1 Accepts the electron pair Supplies the electron pair 2 Electron deficient Electron rich 3 Attacks points of high electron density Attacks points of low electron density 4 Lewis acid Lewis base 5 Possesses an empty orbital to receive electrons Possesses an electron pair which is loosely held and can be supplied easily 6 Usually positively charged species or neutral with empty orbitals Usually negatively charged species or neutral with lone pairs 7 Forms a new bond by accepting electrons Forms a new bond by donating electrons Electronic Effects Four effects influence chemical reactions: Inductive, Mesomeric, Hyperconjugation, Electromeric. 1. Inductive Effect (I-effect) Polarity developed in a carbon chain due to the shifting of $\sigma$ bond electrons by an adjacent group/atom. Discovered by Ingold. Effective up to 3 or 4 carbons, then becomes negligible. Magnitude of I-effect $\propto \frac{1}{\text{distance}}$. Measured relative to hydrogen (I-effect of H is zero). Permanent effect. Negative Inductive (-I) Effect Groups that attract electron density from covalent bonds, acquiring partial negative charge. $$\text{Example: } C^{\delta\delta\delta} \leftarrow C^{\delta\delta} \leftarrow C^{\delta} \leftarrow Z$$ where Z is an electron-withdrawing group. Order of -I effect: $NR_3^+ > -NO_2 > -CN > -SO_3H > -CHO > -COR > -COOH > -COOR > -X > -OH > -OR > -NH_2 > -C_6H_5 > -CH=CH_2 > -H$ Develops positive charge on carbon chain, making it more reactive towards nucleophiles and less reactive towards electrophiles. Positive Inductive (+I) Effect Groups that donate electron density to covalent bonds, acquiring partial positive charge. $$\text{Example: } C^{\delta\delta\delta} \rightarrow C^{\delta\delta} \rightarrow C^{\delta} \rightarrow Z$$ where Z is an electron-donating group. Order of +I effect: $O^- > -COO^- > \text{tert-alkyl} > \text{sec-alkyl} > \text{prim-alkyl} > -CH_3 > -D > -T > -H$ Increases electron density on carbon chain, making it more reactive towards electrophiles and less reactive towards nucleophiles. Magnitude of +I effect $\propto$ size of alkyl group. For isomeric alkyl groups, +I effect $\propto$ number of branches. Application of I-Effect (1) Stability of Carbocation ($R^+$) Stability increases with more +I groups. Stability decreases with more -I groups. Stability of Carbocation $\propto (\text{No. of +I groups}) \propto \frac{1}{(\text{No. of -I groups})}$ Example: $CH_3-CH_2^+ (2) Stability of Carbanion ($R^-$) Stability increases with more -I groups. Stability decreases with more +I groups. Stability of Carbanion $\propto (\text{No. of -I groups}) \propto \frac{1}{(\text{No. of +I groups})}$ Example: $(CH_3)_3C^- (3) Acidic Strength Increases with more -I groups. Decreases with more +I groups. Acidic strength $\propto (\text{No. of -I groups}) \propto \frac{1}{(\text{No. of +I groups})}$ Example: $CH_3COOH Carboxylic acids are generally more acidic than phenols. (4) Basic Strength Increases with more +I groups. Decreases with more -I groups. Basic strength $\propto (\text{No. of +I groups}) \propto \frac{1}{(\text{No. of -I groups})}$ Example: $NH_3 (CH_3)_3N$ (in aqueous solutions, due to solvation effects) Aniline is less basic than alkyl amines due to delocalization of nitrogen's lone pair into the benzene ring. 2. Mesomeric Effect or Resonance Effect (M-effect) Polarity developed in a conjugated system by complete transfer of non-bonding electrons or $\pi$-bond electrons. Involves delocalization of electrons through $p$-orbitals. Permanent effect. Negative Mesomeric (-M) Effect Transfer of $\pi$-bond electrons from the conjugated system to the group. $$\text{Example: In nitroethene, } CH_2=CH-NO_2 \text{, the } NO_2 \text{ group withdraws electrons.}$$ The group has a positive charge or an empty orbital that can accept electrons. Develops positive charge on the conjugated system, decreasing electron density, making it more reactive towards nucleophiles. Groups showing -M effect: $-NO_2, -CN, -SO_3H, -CHO, -COR, -COOH, -COOR, -COX, -CONH_2$. Positive Mesomeric (+M) Effect Transfer of non-bonding electrons from the group to the conjugated system. $$\text{Example: In vinylamine, } CH_2=CH-NH_2 \text{, the } -NH_2 \text{ group donates electrons.}$$ The group has a lone pair of electrons or a negative charge. Develops negative charge on the conjugated system, increasing electron density, making it more reactive towards electrophiles. Groups showing +M effect: $-O^-, -NH_2, -NR_2, -OH, -OR, -SH, -SR, -F, -Cl, -Br, -I$. Resonance Conditions All resonating structures must have the same arrangement of atomic nuclei. Must have the same number of paired and unpaired electrons. Energy of different resonating structures must be similar. All atoms in the delocalization system must be planar or nearly planar. All atoms must obey the octet rule (for major contributors). Conjugation is key: $\pi$ bond adjacent to $\pi$ bond ($C=C-C=C$) $\pi$ bond adjacent to a lone pair ($C=C-\ddot{X}$) $\pi$ bond adjacent to a positive charge ($C=C-C^+$) $\pi$ bond adjacent to a negative charge ($C=C-C^-$) $\pi$ bond adjacent to a free radical ($C=C-C^\cdot$) Characteristics of Resonance Only electrons are transferred, not atoms. Number of electrons (paired/unpaired) must be conserved. Energy of resonating structures is almost same. Permanent effect. All canonical structures must conform to Lewis structures. Resonance Energy Difference between the energy of the most stable canonical structure and the actual molecule (resonance hybrid). It's a measure of extra stability due to resonance. Higher resonance energy = greater resonance stabilization. Benzene resonance energy $\approx 150 \text{ kJ/mol (36 Kcal/mol)}$. Application of M-Effect / Resonance Effect Stability of carbocation: Increased by resonance (e.g., allyl and benzyl carbocations). Aromatic compounds: More stable than non-aromatic compounds due to extensive resonance. Stability order: Benzene Stability of carbanion: Increased by resonance (e.g., allyl and benzyl carbanions). Stability of free radicals: Increased by resonance (e.g., allyl and benzyl radicals). Acidity/Basicity: Resonance can stabilize conjugate bases/acids, affecting their strength. Example: Phenol is more acidic than alcohol due to resonance stabilization of phenoxide ion. 3. Hyperconjugation Effect (H-effect) Complete transfer of $\sigma$ bond electrons of C-H to an adjacent $\pi$ bond, positive charge, or free electron. Also called non-bonded resonance or Baker-Nathan effect. Permanent effect. Conditions for H-effect Presence of $\alpha$-hydrogens (hydrogens on carbon adjacent to a multiple bond, positive charge, or free radical). The molecule must have a C-H $\sigma$ bond adjacent to: a $\pi$ bond (alkenes, alkynes). a positive charge (carbocations). a free electron (free radicals). Application of H-Effect Stability of carbocation: More hyperconjugation structures (more $\alpha$-H) = more stable carbocation. $$\text{Stability of carbocation } \propto (\text{No. of canonical structures}) \propto (\text{No. of } \alpha\text{-H})$$ Example: $CH_3^+ Stability of carbon free radicals: More hyperconjugation structures (more $\alpha$-H) = more stable free radical. Stability of alkenes: More hyperconjugation structures (more $\alpha$-H) = more stable alkene. Example: $CH_2=CH_2 Heat of hydrogenation: Inversely proportional to the stability of the alkene. $$\text{Heat of hydrogenation } \propto \frac{1}{\text{stability of alkene}} \propto \frac{1}{\text{number of } \alpha\text{-H}}$$ Reactivity of benzene: Alkyl groups attached to benzene activate the ring towards electrophilic substitution due to +I and hyperconjugation. 4. Electromeric Effect (E-effect) Temporary effect in organic compounds with multiple bonds (double or triple). Occurs only in the presence of an attacking reagent. Complete transfer of a shared pair of $\pi$-electrons to one of the atoms joined by the multiple bond. Annulled once the attacking reagent is removed. Positive Electromeric (+E) Effect $\pi$-electrons are transferred to the atom to which the reagent gets attached. $$\text{Example: } >C=C C^+-C-H$$ The $\pi$ electrons move towards the carbon that bonds to $H^+$. Negative Electromeric (-E) Effect $\pi$-electrons are transferred to the atom to which the attacking reagent does NOT get attached. $$\text{Example: } >C=O \xrightarrow{CN^-} >C-O^-$$ $$ \qquad \qquad \qquad \qquad \quad \quad |$$ $$ \qquad \qquad \qquad \qquad \quad \quad CN$$ The $\pi$ electrons move towards oxygen, while $CN^-$ attacks carbon. Aromaticity Property of cyclic, conjugated compounds with unusual stability due to delocalized $\pi$ electrons. Benzene: Planar, cyclic, 6 $\pi$ electrons (3 pairs), all C-C bonds have equal length (1.39 Å), intermediate between single (1.54 Å) and double (1.34 Å). Hückel's Rule: For a planar, cyclic compound to be aromatic, its uninterrupted $\pi$ cloud must contain $(4n+2)$ $\pi$ electrons, where $n$ is a whole number $(0, 1, 2, ...)$. Aromatic compounds must have an odd number of pairs of $\pi$ electrons (e.g., 2, 6, 10, 14...). Anti-aromatic compounds: Cyclic, planar, fully conjugated systems with $4n$ $\pi$ electrons (an even number of $\pi$ pairs). These are highly unstable and often distort to avoid planarity. (e.g., cyclobutadiene, cyclooctatetraene in planar form). Non-aromatic compounds: Compounds that are not cyclic, not planar, or not fully conjugated. They do not exhibit special stability. (e.g., cyclohexene, cyclooctatetraene in tub conformation). Heterocyclic aromatic compounds: Cyclic compounds with atoms other than carbon in the ring that contribute to the $\pi$ system (e.g., Pyridine, Pyrrole, Furan, Thiophene). Reaction Intermediates Transient species formed during a reaction, often highly reactive. 1. Carbocation (Carbenium ion) Positively charged carbon atom. Contains only six electrons in three bonds (electron deficient). Acts as an electrophile and Lewis acid. Structure: $sp^2$ hybridized, trigonal planar, with an unhybridized vacant 'p' orbital perpendicular to the plane. Stability: Stabilized by +I effect, +M effect, and hyperconjugation. Order of stability: $3^\circ > 2^\circ > 1^\circ > CH_3^+$. Allyl and benzyl carbocations are highly stable due to resonance. Generation: Heterolytic cleavage of C-X bond (e.g., $R_3C-Cl \rightarrow R_3C^+ + Cl^-$). Protonation of unsaturated compounds (e.g., $CH_2=CH_2 + H^+ \rightarrow CH_3CH_2^+$). Protonation of alcohols followed by water loss (e.g., $R-OH + H^+ \rightarrow R-OH_2^+ \rightarrow R^+ + H_2O$). Decomposition of diazonium salts. Reactions: Proton Loss (to form alkenes). Combination with Nucleophiles (to form substitution products). Addition to alkenes (polymerization). Molecular Rearrangement (hydride or alkyl shifts to form a more stable carbocation). 2. Carbanion Carbon intermediate with three bond pairs and a negative charge (a lone pair of electrons). Contains eight electrons in its valence shell. Acts as a nucleophile and Lewis base. Structure: Typically $sp^3$ hybridized (pyramidal geometry) if the negative charge is localized. If the negative charge is conjugated with a $\pi$ system, it can be $sp^2$ hybridized (planar). Stability: Stabilized by electron-withdrawing effects (-I, -M), delocalization of charge, and aromatization. Order of stability: $CH_3^- > 1^\circ > 2^\circ > 3^\circ$. Allyl and benzyl carbanions are stable due to resonance. Generation: Action of strong base on acidic C-H bond (e.g., $CH_3COCH_3 + NaOH \rightarrow CH_3COCH_2^- Na^+$). Reaction of organometallic compounds (e.g., $R-Mg-X \rightarrow R^-$). Reactions: Attachment of $H^+$ (protonation). Loss of leaving group (to form $\pi$ bond, elimination). Nucleophilic addition reactions. Nucleophilic substitution reactions. 3. Carbon Free Radical Species with an unpaired electron on a carbon atom. Contains seven electrons in its valence shell. Highly reactive. Structure: Can be $sp^3$ hybridized (pyramidal) or $sp^2$ hybridized (planar). Planar is generally preferred for simple alkyl radicals. Stability: Stabilized by M-effect and H-effect. More hyperconjugation structures ($\alpha$-H) = more stable. Order of stability: $3^\circ > 2^\circ > 1^\circ > CH_3^\cdot$. Allyl and benzyl radicals are highly stable due to resonance. Generation: Homolytic cleavage of a covalent bond (e.g., $Cl-Cl \xrightarrow{hv} 2Cl^\cdot$). Decomposition of peroxides (e.g., $R-O-O-R \rightarrow 2R-O^\cdot$). From azo compounds (e.g., $R-N=N-R \rightarrow 2R^\cdot + N_2$). Reactions: Recombination (coupling) with another radical. Disproportionation (transfer of H atom between two radicals). Abstraction of an atom (e.g., $R^\cdot + HCl \rightarrow RH + Cl^\cdot$). Addition to unsaturated compounds (polymerization). 4. Carbene Neutral species with a carbon atom forming two bonds and having two non-bonded electrons. Contains only six electrons in its valence shell (electron deficient). Acts as an electrophile. Types: Singlet Carbene: Two non-bonded electrons in the same $sp^2$ hybridized orbital with opposite spin. Bent structure. Diamagnetic. More electrophilic. Triplet Carbene: Two non-bonded electrons in two different 'p' orbitals with parallel spin. Linear structure. Paramagnetic. Less selective but more reactive in some reactions. Generation: Decomposition of diazomethane ($CH_2N_2 \xrightarrow{hv} :CH_2 + N_2$). Photolysis of ketene ($CH_2=C=O \xrightarrow{hv} :CH_2 + CO$). Alpha-elimination of chloroform with a strong base ($CHCl_3 + KOH \rightarrow :CCl_2 + KCl + H_2O$). Reactions: Addition to alkenes (cyclopropanation, e.g., Simmons-Smith reaction). Insertion reactions into C-H bonds. 5. Benzyne Highly reactive, neutral intermediate derived from benzene by removing two ortho-hydrogens and forming an additional "triple bond" within the ring. The "triple bond" is highly strained, consisting of a normal $\pi$ bond and a weak, distorted $\pi$ bond formed by sideways overlap of $sp^2$ orbitals. All carbon atoms are $sp^2$ hybridized. Generation: Treatment of aryl halides (especially bromobenzene or chlorobenzene) with strong bases (e.g., $NaNH_2$ in liquid $NH_3$). $$\text{Example: } C_6H_5Br + NaNH_2 \xrightarrow{liq. NH_3} \text{Benzyne intermediate}$$ Thermal decomposition of 1,2-disubstituted benzene derivatives. Reactions: Extremely reactive due to strain. Reacts readily with nucleophiles (e.g., $NH_2^-$) leading to substitution product. Can undergo Diels-Alder reactions. 6. Nitrene Electron-deficient species with a sextet of electrons on nitrogen, analogous to carbene. General formula: $R-\ddot{N}:$. Can exist as singlet or triplet states similar to carbenes. Generation: Thermal or photochemical decomposition of azides ($R-N_3 \xrightarrow{hv \text{ or } \Delta} R-\ddot{N}: + N_2$). Hofmann rearrangement of amides. Loss of $CO$ from isocyanates. Reactions: Insertion reactions into C-H bonds. Addition to alkenes to form aziridines. Rearrangement reactions (e.g., Hofmann, Curtius, Schmidt rearrangements). Types of Reactions Organic reactions are broadly classified into four main types: 1. Substitution Reaction An atom or group is replaced by another atom or group. (a) Electrophilic Substitution Reactions (ESR) Characteristic reaction of aromatic compounds (e.g., benzene). Electrophile replaces a hydrogen atom on the ring. Nitration: $$C_6H_6 + HNO_3 \xrightarrow{conc. H_2SO_4} C_6H_5NO_2 + H_2O$$ Halogenation: $$C_6H_6 + Cl_2 \xrightarrow{FeCl_3} C_6H_5Cl + HCl$$ Friedel-Crafts Alkylation: $$C_6H_6 + RCl \xrightarrow{AlCl_3} C_6H_5R + HCl$$ Friedel-Crafts Acylation: $$C_6H_6 + RCOCl \xrightarrow{AlCl_3} C_6H_5COR + HCl$$ Gatterman-Koch Aldehyde Synthesis: $$C_6H_6 + CO + HCl \xrightarrow{AlCl_3/CuCl} C_6H_5CHO + HCl$$ (b) Nucleophilic Substitution Reactions ($S_N$) Common for alkyl halides and other compounds with good leaving groups. A nucleophile replaces a leaving group. $S_N2$ (Bimolecular Nucleophilic Substitution): $$Nu^- + R-X \rightarrow R-Nu + X^-$$ (e.g., $OH^- + CH_3Br \rightarrow CH_3OH + Br^-$) $S_N1$ (Unimolecular Nucleophilic Substitution): $$R-X \rightarrow R^+ + X^- \quad (\text{slow})$$ $$R^+ + Nu^- \rightarrow R-Nu \quad (\text{fast})$$ (e.g., $(CH_3)_3C-Cl + H_2O \rightarrow (CH_3)_3C-OH + HCl$) (c) Named Substitution Reactions Wurtz Reaction: Coupling of alkyl halides to form alkanes. $$2R-X + 2Na \xrightarrow{dry \text{ ether}} R-R + 2NaX$$ Wurtz-Fittig Reaction: Coupling of an alkyl halide and an aryl halide to form an alkylbenzene. $$Ar-X + R-X + 2Na \xrightarrow{dry \text{ ether}} Ar-R + 2NaX$$ Fittig Reaction: Coupling of two aryl halides to form a biaryl. $$2Ar-X + 2Na \xrightarrow{dry \text{ ether}} Ar-Ar + 2NaX$$ Ullmann Reaction: Similar to Fittig, coupling of aryl halides using copper. $$2Ar-X + Cu \xrightarrow{\Delta} Ar-Ar + CuX_2$$ Sandmeyer Reaction: Conversion of aryl diazonium salts to aryl halides or cyanides. $$Ar-N_2^+ X^- \xrightarrow{CuX \text{ or } CuCN} Ar-X \text{ or } Ar-CN + N_2$$ Gattermann Reaction: Similar to Sandmeyer, but uses copper powder and HX for aryl halides. $$Ar-N_2^+ X^- \xrightarrow{Cu \text{ powder}/HX} Ar-X + N_2$$ Gattermann Reaction (Formylation): Formylation of activated aromatic rings. $$Ar-H + HCN + HCl \xrightarrow{AlCl_3} Ar-CHO + HCl$$ Kolbe's Reaction (Kolbe-Schmitt Reaction): Carboxylation of phenol to form salicylic acid. $$C_6H_5OH + CO_2 \xrightarrow{NaOH, \Delta, \text{ pressure}} o-HOC_6H_4COOH$$ Reimer-Tiemann Reaction: Formylation of phenol to form salicylaldehyde. $$C_6H_5OH + CHCl_3 + 3NaOH \xrightarrow{\Delta} o-HOC_6H_4CHO + 3NaCl + 2H_2O$$ Hell-Volhard-Zelinsky (HVZ) Reaction: Halogenation of $\alpha$-carbon of carboxylic acids. $$RCH_2COOH + X_2 \xrightarrow{Red P} RCHXCOOH + HX$$ 2. Elimination Reactions (E) Removal of two atoms or groups from adjacent carbons, forming a double or triple bond (often called dehydrohalogenation, dehydration, etc.). (a) $E2$ (Bimolecular Elimination) One-step concerted mechanism. $$R_2CH-CR_2X + Base \rightarrow R_2C=CR_2 + H-Base^+ + X^-$$ (e.g., $CH_3CH_2Br + KOH \xrightarrow{alc.} CH_2=CH_2 + KBr + H_2O$) Saytzeff Rule: For neutral substrates, the more substituted alkene (more stable) is the major product. Hofmann Rule: If the leaving group is bulky (e.g., $NR_3^+$) or fluorine, the less substituted alkene (less hindered) is the major product. (b) $E1$ (Unimolecular Elimination) Two-step mechanism: 1) formation of carbocation (rate-determining step), 2) removal of a proton by a weak base. $$R-X \rightarrow R^+ + X^-$$ $$R^+ + Base \rightarrow \text{Alkene} + H-Base^+$$ (e.g., $(CH_3)_3C-Cl \xrightarrow{H_2O, \Delta} (CH_3)_2C=CH_2 + HCl$) (c) Named Elimination Reactions Hofmann Elimination: Exhaustive methylation of amines followed by heating to form an alkene. $$R_2CH-CR_2-N(CH_3)_3^+ OH^- \xrightarrow{\Delta} R_2C=CR_2 + (CH_3)_3N + H_2O$$ Chugaev Elimination: Pyrolytic syn-elimination of xanthates to form alkenes. $$R-CH_2-CH_2-OH \xrightarrow{CS_2, NaOH} R-CH_2-CH_2-OCS_2Na \xrightarrow{CH_3I} R-CH_2-CH_2-OCS_2CH_3 \xrightarrow{\Delta} R-CH=CH_2 + COS + CH_3SH$$ 3. Addition Reaction Reagents add across a multiple bond (double or triple bond) to form a single product, reducing the degree of unsaturation. (a) Electrophilic Addition (EA) Initiated by an electrophile attacking the $\pi$ bond of an alkene or alkyne. $$\text{Example: } CH_2=CH_2 + HBr \rightarrow CH_3CH_2Br$$ Markovnikov's Rule: In the addition of HX to unsymmetrical alkenes, the hydrogen atom adds to the carbon atom of the double bond that already has more hydrogen atoms. $$CH_3-CH=CH_2 + HBr \rightarrow CH_3-CHBr-CH_3 \quad (\text{major})$$ (b) Nucleophilic Addition (NA) Characteristic of aldehydes and ketones (carbonyl compounds). $$RCHO + HCN \rightarrow RCH(OH)CN$$ Aldol Condensation: Aldehydes/ketones with $\alpha$-hydrogens react to form $\beta$-hydroxy carbonyl compounds. $$2CH_3CHO \xrightarrow{dil. NaOH} CH_3CH(OH)CH_2CHO$$ Cross-Aldol Condensation: Between two different aldehydes/ketones. Claisen-Schmidt Condensation: Aldol condensation between an aromatic aldehyde and an aliphatic aldehyde/ketone. Perkin Reaction: Aromatic aldehydes with acid anhydrides in the presence of a weak base to give $\alpha, \beta$-unsaturated carboxylic acids. $$ArCHO + (RCH_2CO)_2O \xrightarrow{RCH_2COONa} ArCH=CRCOOH$$ Knoevenagel Condensation: Aldehydes/ketones with active methylene compounds (e.g., malonic ester). $$R_2C=O + CH_2(COOR')_2 \xrightarrow{Base} R_2C=C(COOR')_2$$ Favorskii Reaction: Addition of terminal alkynes to carbonyl compounds. $$R-C \equiv CH + R'R''C=O \xrightarrow{Base} R-C \equiv C-CR'R''OH$$ Darzens Glycidic Ester Condensation: Aldehydes/ketones with $\alpha$-halo esters to form $\alpha, \beta$-epoxy esters. $$R_2C=O + XCH_2COOR' \xrightarrow{Base} \text{Epoxy ester}$$ Benzoin Condensation: Aromatic aldehydes with cyanide catalyst to form $\alpha$-hydroxy ketones. $$2ArCHO \xrightarrow{KCN} ArCH(OH)COAr$$ Michael Addition: Addition of a carbanion (from active methylene compound) to an $\alpha, \beta$-unsaturated carbonyl compound. $$R-\ddot{C}H_2 + R'CH=CH-COR'' \rightarrow R'-CH(R)-CH_2-COR''$$ Wittig Reaction: Aldehydes/ketones with phosphorus ylides to form alkenes. $$R_2C=O + Ph_3P=CR'_2 \rightarrow R_2C=CR'_2 + Ph_3P=O$$ Reformatsky Reaction: Aldehydes/ketones with $\alpha$-halo esters in the presence of zinc to form $\beta$-hydroxy esters. $$R_2C=O + BrCH_2COOR' \xrightarrow{Zn} R_2C(OH)CH_2COOR'$$ Mannich Reaction: Condensation of an aldehyde, a primary or secondary amine, and a compound with an acidic $\alpha$-hydrogen. $$R_2C=O + R'_2NH + CH_2(COOH)_2 \rightarrow \text{Mannich base}$$ Dieckmann Condensation: Intramolecular Claisen condensation of diesters to form cyclic $\beta$-keto esters. $$\text{Diester} \xrightarrow{Base} \text{Cyclic } \beta\text{-keto ester}$$ (c) Free Radical Addition (FRA) Initiated by a free radical. $$CH_2=CH_2 + HBr \xrightarrow{Peroxide} CH_3CH_2Br \quad (\text{Anti-Markovnikov})$$ 4. Rearrangement Reaction A reaction where the skeletal structure of a molecule is rearranged to form a structural isomer of the original molecule. Often involves migration of an atom or group. Pinacol-Pinacolone Rearrangement: Vicinal diols to ketones/aldehydes, typically acid-catalyzed. $$(CH_3)_2C(OH)-C(OH)(CH_3)_2 \xrightarrow{H_2SO_4} (CH_3)_3C-CO-CH_3$$ Beckmann Rearrangement: Oximes to amides, acid-catalyzed. $$R_2C=NOH \xrightarrow{H_2SO_4} RCONHR$$ Baeyer-Villiger Rearrangement: Ketones to esters (or cyclic ketones to lactones) using peroxy acids. $$R_2C=O + R'CO_3H \rightarrow RCOOR + R'COOH$$ Hofmann Rearrangement: Primary amides to primary amines with one less carbon atom. $$RCONH_2 + Br_2 + 4NaOH \rightarrow RNH_2 + Na_2CO_3 + 2NaBr + 2H_2O$$ Curtius Rearrangement: Acyl azides to isocyanates, then to amines or urethanes. $$RCON_3 \xrightarrow{\Delta} RN=C=O + N_2 \xrightarrow{H_2O} RNH_2 + CO_2$$ Schmidt Rearrangement: Carboxylic acids or ketones with hydrazoic acid ($HN_3$) to amines or amides. $$RCOOH + HN_3 \xrightarrow{H_2SO_4} RNH_2 + N_2 + CO_2$$ $$R_2C=O + HN_3 \xrightarrow{H_2SO_4} RCONHR$$ Claisen Rearrangement: Thermal rearrangement of allyl vinyl ethers to $\gamma,\delta$-unsaturated carbonyl compounds. $$\text{Allyl vinyl ether} \xrightarrow{\Delta} \gamma,\delta\text{-unsaturated carbonyl compound}$$ Fries Rearrangement: Phenyl esters to $o$- and $p$-hydroxyketones using Lewis acid catalyst. $$Ar-O-COR \xrightarrow{AlCl_3} o/p-HOC_6H_4COR$$ Favorskii Rearrangement: $\alpha$-halo ketones to carboxylic acids or esters in the presence of base. $$\text{Cyclic } \alpha\text{-halo ketone} \xrightarrow{Base} \text{Carboxylic acid/ester (ring contraction)}$$ Wolff Rearrangement: $\alpha$-diazo ketones to ketenes, which then react with nucleophiles to form carboxylic acid derivatives. $$RCOCHN_2 \xrightarrow{Ag_2O, \Delta} RCH=C=O + N_2 \xrightarrow{H_2O} RCH_2COOH$$ Benzilic Acid Rearrangement: $\alpha$-diketones to $\alpha$-hydroxy acids with strong base. $$ArCOCOAr \xrightarrow{KOH, H_2O} Ar_2C(OH)COOH$$ Cannizzaro Reaction: Disproportionation of non-enolizable aldehydes (no $\alpha$-hydrogens) in the presence of strong base. $$2ArCHO \xrightarrow{conc. NaOH} ArCOOH + ArCH_2OH$$ Tischenko Reaction: Aldehydes to esters using an aluminium alkoxide catalyst. $$2RCHO \xrightarrow{Al(OR')_3} RCOOCH_2R$$ Meerwein-Ponndorf-Verley (MPV) Reduction: Reduction of aldehydes and ketones to alcohols using an aluminum alkoxide and a secondary alcohol. $$R_2C=O + (CH_3)_2CHOH \xrightarrow{Al(O-i-Pr)_3} R_2CHOH + (CH_3)_2C=O$$ Oppenauer Oxidation: Oxidation of secondary alcohols to ketones using an aluminum alkoxide and a ketone. $$R_2CHOH + R'_2C=O \xrightarrow{Al(O-t-Bu)_3} R_2C=O + R'_2CHOH$$