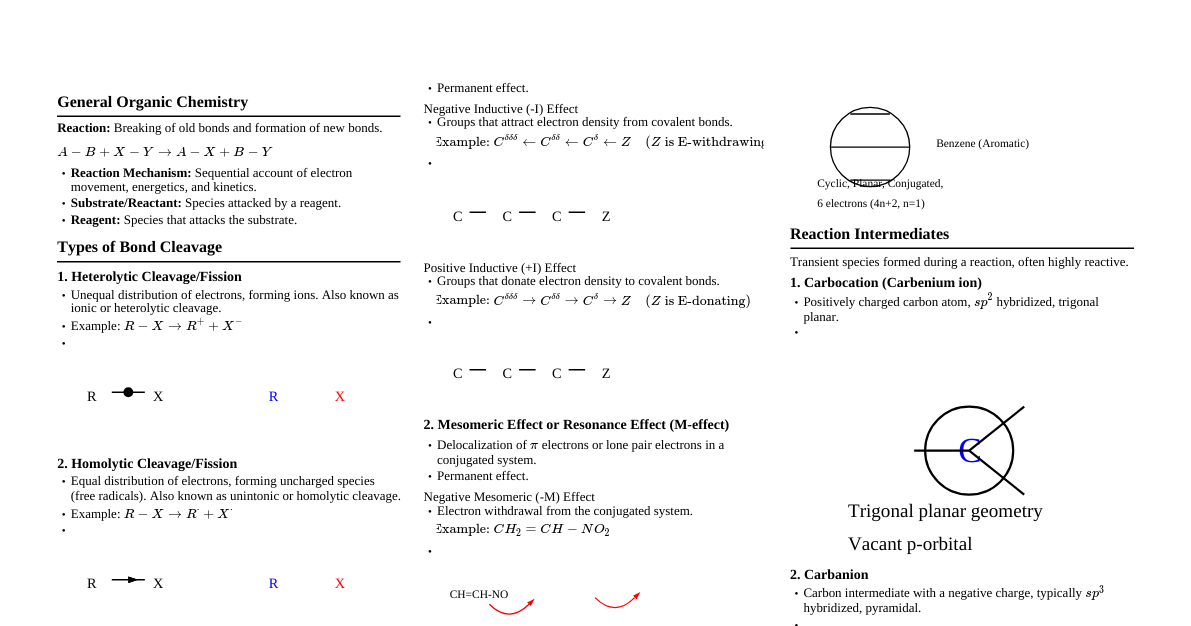
1. Atomic Structure & Bonding 1.1 Atomic Orbitals s-orbital: Spherical, 1 orbital per shell. p-orbital: Dumbbell-shaped, 3 orbitals per shell (px, py, pz). d-orbital: More complex shapes, 5 orbitals per shell. Electrons fill lowest energy orbitals first (Aufbau principle). Pauli Exclusion Principle: Max 2 electrons per orbital, opposite spins. Hund's Rule: Degenerate orbitals fill singly before pairing. 1.2 Hybridization $sp^3$: 4 single bonds (tetrahedral, $109.5^\circ$), e.g., Methane ($CH_4$). $sp^2$: 1 double bond, 2 single bonds (trigonal planar, $120^\circ$), e.g., Ethene ($C_2H_4$). $sp$: 1 triple bond, 1 single bond OR 2 double bonds (linear, $180^\circ$), e.g., Ethyne ($C_2H_2$). Determined by counting $\sigma$ bonds + lone pairs around central atom. 1.3 Types of Bonds Ionic: Transfer of electrons, large electronegativity difference. Covalent: Sharing of electrons. Nonpolar Covalent: Equal sharing, similar electronegativity. Polar Covalent: Unequal sharing, moderate electronegativity difference ($\delta^+$, $\delta^-$). Bond Length: $C \equiv C Bond Strength: $C \equiv C > C = C > C - C$. 2. Isomerism 2.1 Constitutional Isomers Same molecular formula, different connectivity. Chain Isomers: Different carbon skeletons (e.g., n-butane vs. isobutane). Positional Isomers: Same carbon skeleton, different position of functional group/substituent (e.g., 1-propanol vs. 2-propanol). Functional Group Isomers: Different functional groups (e.g., ethanol vs. dimethyl ether). 2.2 Stereoisomers Same molecular formula, same connectivity, different spatial arrangement. Enantiomers: Non-superimposable mirror images. Requires chiral center(s). Diastereomers: Stereoisomers that are NOT mirror images. Geometric (cis/trans) Isomers: Restricted rotation (double bonds, rings), substituents on same (cis) or opposite (trans) sides. Conformational Isomers: Differ by rotation around single bonds (e.g., staggered vs. eclipsed ethane). 2.3 Chirality A molecule is chiral if it is non-superimposable on its mirror image. Chiral center: Carbon atom bonded to four different groups. R/S Configuration: Assign priorities (atomic number) to groups attached to chiral center. Orient molecule so lowest priority group (4) is pointing away. Trace path from 1 to 2 to 3. Clockwise = R, Counter-clockwise = S. Meso Compound: Contains chiral centers but is achiral due to an internal plane of symmetry. 3. Functional Groups & IUPAC Nomenclature 3.1 IUPAC Priority Order for Functional Groups Carboxylic Acids ($R-COOH$) Esters ($R-COOR'$) Acyl Halides ($R-COX$) Amides ($R-CONH_2$) Nitriles ($R-C \equiv N$) Aldehydes ($R-CHO$) Ketones ($R-CO-R'$) Alcohols ($R-OH$) Phenols (Ar-OH) Thiols ($R-SH$) Amines ($R-NH_2$) Imines ($R_2C=NR'$) Alkenes ($C=C$) Alkynes ($C \equiv C$) Ethers ($R-O-R'$) Haloalkanes ($R-X$) Nitro compounds ($R-NO_2$) Alkanes ($R-H$) 3.2 Common Functional Groups and IUPAC Naming Group Name Structure Description Suffix (Main FG) Prefix (Substituent) Alkane $R-H$ Hydrocarbons with only single C-C bonds. Saturated. -ane alkyl- Alkene $R_2C=CR_2$ Hydrocarbons with at least one C=C double bond. Unsaturated. -ene alkenyl- Alkyne $R-C \equiv C-R'$ Hydrocarbons with at least one C≡C triple bond. Unsaturated. -yne alkynyl- Arene (Aromatic) Benzene ring and derivatives Cyclic, planar, conjugated systems with $(4n+2)\pi$ electrons. -benzene phenyl-, benzyl- Haloalkane $R-X$ (X = F, Cl, Br, I) Alkanes with one or more hydrogen atoms replaced by halogens. - fluoro-, chloro-, bromo-, iodo- Alcohol $R-OH$ Contains a hydroxyl group (-OH) bonded to an aliphatic carbon. -ol hydroxy- Phenol Ar-OH Contains a hydroxyl group (-OH) bonded directly to an aromatic ring. -phenol hydroxy- Ether $R-O-R'$ Contains an oxygen atom bonded to two alkyl or aryl groups. - alkoxy- (e.g., methoxy-, ethoxy-) Thiol $R-SH$ Sulfur analog of alcohol, contains a sulfhydryl group (-SH). -thiol mercapto- Amine $R-NH_2$, $R_2NH$, $R_3N$ Organic derivatives of ammonia, containing nitrogen bonded to alkyl/aryl groups. -amine amino-, methylamino- etc. Aldehyde $R-CHO$ Contains a carbonyl group (C=O) bonded to a hydrogen and an alkyl/aryl group. -al formyl-, oxo- Ketone $R-CO-R'$ Contains a carbonyl group (C=O) bonded to two alkyl or aryl groups. -one oxo- Carboxylic Acid $R-COOH$ Contains a carboxyl group (-COOH), which is a carbonyl and a hydroxyl group. Acidic. -oic acid carboxy- Ester $R-COO-R'$ Derivative of carboxylic acid where the -OH is replaced by -OR'. Formed from alcohol + acid. -oate alkoxycarbonyl- Acyl Halide $R-COX$ Derivative of carboxylic acid where the -OH is replaced by a halogen (-X). -oyl halide halocarbonyl- Amide $R-CONH_2$ Derivative of carboxylic acid where the -OH is replaced by an amino group (-NH2, -NHR, -NR2). -amide carbamoyl- Nitrile $R-C \equiv N$ Contains a cyano group (-C≡N). Triple bond between C and N. -nitrile cyano- Nitro Compound $R-NO_2$ Contains a nitro group (-NO2) bonded to an alkyl or aryl group. - nitro- Sulfide (Thioether) $R-S-R'$ Sulfur analog of ether, contains a sulfur atom bonded to two alkyl or aryl groups. - alkylthio- Sulfonic Acid $R-SO_3H$ Contains a sulfonyl hydroxyl group. Strong acid. -sulfonic acid sulfo- 3.3 IUPAC Naming Rules Summary Parent Chain: Find longest continuous carbon chain containing the highest priority functional group. Numbering: Number the parent chain to give the highest priority functional group the lowest possible number. If a tie, give substituents the lowest numbers. Substituents: Identify and name all substituents. Alphabetization: List substituents alphabetically (ignoring prefixes like di-, tri-, sec-, tert-). Combine: Position numbers, prefixes (if any), substituent names, parent chain name, and functional group suffix. Punctuation: Hyphens for numbers-words, commas for numbers-numbers. 4. Reaction Mechanisms 4.1 Nucleophiles & Electrophiles Nucleophile: Electron-rich species, donates electron pair (Lewis base). Often negatively charged or has lone pair. (e.g., $OH^-$, $H_2O$, $NH_3$). Electrophile: Electron-deficient species, accepts electron pair (Lewis acid). Often positively charged or has partial positive charge. (e.g., $H^+$, $Br^+$, carbonyl carbon). 4.2 Types of Reactions Substitution: One atom/group replaced by another. Nucleophilic Substitution ($SN1$, $SN2$). Electrophilic Aromatic Substitution ($EAS$). Addition: Atoms/groups added across a double/triple bond. Electrophilic Addition (e.g., $HBr$ to alkene). Nucleophilic Addition (e.g., to carbonyls). Elimination: Atoms/groups removed, forming a double/triple bond. $E1$, $E2$. Rearrangement: Atoms/groups migrate within a molecule. 4.3 Acid-Base Chemistry Brønsted-Lowry: Acid donates $H^+$, Base accepts $H^+$. Lewis: Acid accepts electron pair, Base donates electron pair. $pK_a$: Measure of acid strength. Lower $pK_a$ = stronger acid. Factors affecting acidity: Electronegativity, resonance, inductive effect, hybridization. 5. Spectroscopy 5.1 Infrared (IR) Spectroscopy Identifies functional groups based on vibrational frequencies. Key absorptions: $O-H$ (alcohols): Broad, strong, $3200-3600 cm^{-1}$ $N-H$ (amines): Medium, $3300-3500 cm^{-1}$ (1 or 2 peaks) $C=O$ (carbonyls): Strong, $1650-1780 cm^{-1}$ $C \equiv C$ (alkynes): Weak, $2100-2260 cm^{-1}$ $C \equiv N$ (nitriles): Medium, $2210-2260 cm^{-1}$ $C-H$ (alkane): Strong, $2850-2960 cm^{-1}$ $C-H$ (alkene): $3020-3100 cm^{-1}$ $C-H$ (aromatic): $3030 cm^{-1}$ 5.2 Nuclear Magnetic Resonance (NMR) Spectroscopy Identifies carbon-hydrogen framework. $^1H$ NMR: Chemical Shift ($\delta$): Position of signal (ppm), indicates electronic environment. $0.9$ (methyl), $1.2$ (methylene), $1.5$ (methine) $2.0-2.5$ (allylic, benzylic, $C=O$ adjacent) $3.5-4.0$ (next to $O$, $N$, $X$) $4.5-6.0$ (vinyl H) $6.5-8.5$ (aromatic H) $9.0-10.0$ (aldehyde H) $10.0-13.0$ (carboxylic acid H) Integration: Area under peak, proportional to number of equivalent protons. Multiplicity (Splitting): $(n+1)$ rule, where $n$ is number of equivalent adjacent protons. (e.g., singlet, doublet, triplet, quartet). $^{13}C$ NMR: Shows number of unique carbon environments. Chemical shifts range $0-220$ ppm. No splitting usually observed (proton decoupled). 5.3 Mass Spectrometry (MS) Determines molecular weight and molecular formula. Molecular Ion ($M^+$): Peak with highest m/z, corresponds to molecular weight. Base Peak: Most intense peak, represents most stable fragment. Fragment patterns provide structural information. $(M+1)^+$ peak: Due to presence of $^{13}C$. $(M+2)^+$ peak: Significant for $Cl$ (1:3 ratio with $M^+$) and $Br$ (1:1 ratio with $M^+$). 6. Reaction Types & Reagents (Examples) 6.1 Alkanes Free Radical Halogenation: $R-H + X_2 \xrightarrow{hv/\Delta} R-X + HX$. 6.2 Alkenes Hydrogenation: $R_2C=CR_2 + H_2 \xrightarrow{Pd/Pt/Ni} R_2CH-CHR_2$ (syn addition). Halogenation: $R_2C=CR_2 + X_2 \rightarrow R_2CX-CXR_2$ (anti addition). Hydrohalogenation: $R_2C=CR_2 + HX \rightarrow R_2CH-CXR_2$ (Markovnikov). Hydration: $R_2C=CR_2 + H_2O \xrightarrow{H_2SO_4} R_2CH-CR_2OH$ (Markovnikov). Oxidation (OsO$_4$): Forms syn-diols. Ozonolysis ($O_3, H_2O_2$): Cleaves double bond, forms aldehydes/ketones. 6.3 Alkynes Hydrogenation: $R-C \equiv C-R' + H_2 \xrightarrow{Pd/Pt/Ni} R-CH_2-CH_2-R'$. Partial Hydrogenation (Lindlar's catalyst): Forms cis-alkenes. Partial Hydrogenation (Na, $NH_3$): Forms trans-alkenes. Hydrohalogenation: $R-C \equiv C-R' + HX \rightarrow R-CH=CX-R'$. 6.4 Alcohols Oxidation: Primary alcohol $\xrightarrow{PCC}$ Aldehyde. Primary alcohol $\xrightarrow{CrO_3/H_2SO_4}$ Carboxylic acid. Secondary alcohol $\xrightarrow{CrO_3/H_2SO_4}$ Ketone. Dehydration: Alcohol $\xrightarrow{H_2SO_4/\Delta}$ Alkene (Zaitsev's rule). Substitution with $HX$: $R-OH + HX \rightarrow R-X + H_2O$. 6.5 Carbonyl Compounds (Aldehydes & Ketones) Nucleophilic Addition: Grignard Reagent ($RMgX$): Adds $R$ group, forms alcohol. Hydride Reduction ($NaBH_4, LiAlH_4$): Aldehyde $\rightarrow$ Primary alcohol; Ketone $\rightarrow$ Secondary alcohol. Cyanohydrin Formation ($HCN$): Forms $\alpha$-hydroxynitriles. Oxidation: Aldehyde $\xrightarrow{CrO_3/H_2SO_4}$ Carboxylic acid. (Ketones generally resist oxidation). 6.6 Carboxylic Acids & Derivatives Esterification: $R-COOH + R'-OH \xrightarrow{H^+}$ $R-COO-R'$. Reduction ($LiAlH_4$): $R-COOH \rightarrow R-CH_2OH$. Amide formation: $R-COOH + R'-NH_2 \xrightarrow{\Delta}$ $R-CONHR'$.