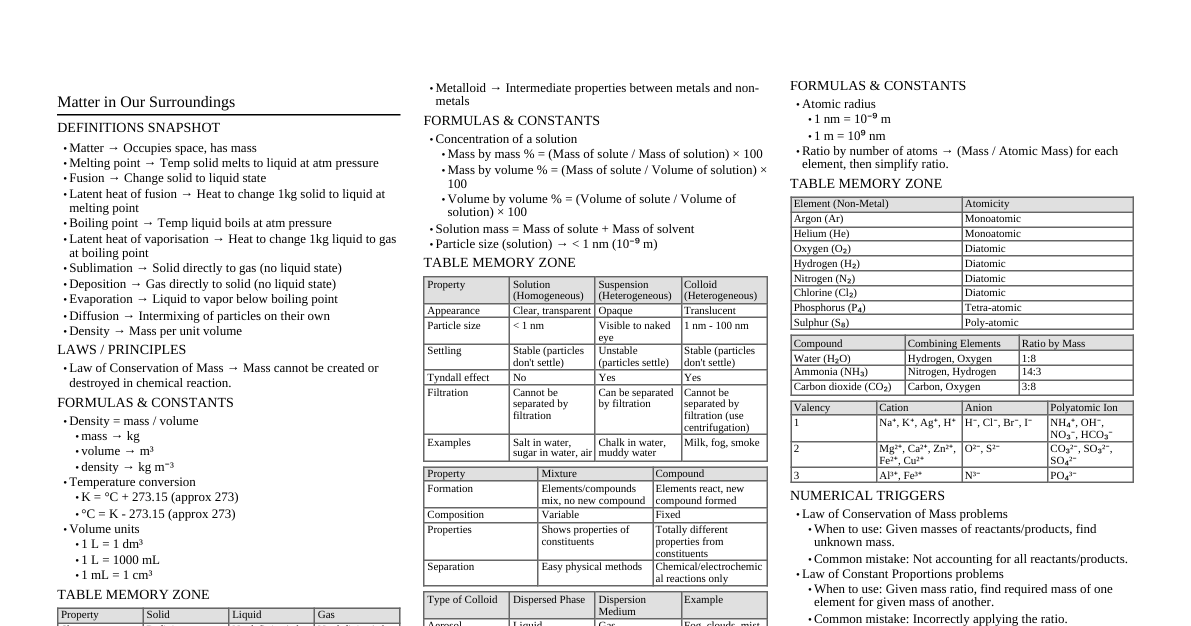
### Iodine Extraction - **Main Source:** Seaweeds (e.g., kelp, Fucus, Laminaria). - **Extraction from Seaweeds:** 1. Seaweeds are dried and burnt to form ash called "kelp." 2. Kelp contains about 0.5-1.5% iodine as iodides (NaI, KI). 3. Water is added to dissolve iodides, and the solution is concentrated. 4. The concentrated solution is treated with MnO₂ and H₂SO₄, which oxidizes iodides to iodine: $$2NaI + MnO_2 + 2H_2SO_4 \rightarrow Na_2SO_4 + MnSO_4 + 2H_2O + I_2$$ Or, by passing Cl₂ gas: $$2NaI + Cl_2 \rightarrow 2NaCl + I_2$$ ### First Order Reaction - **Definition:** A reaction whose rate depends on the concentration of only one reactant raised to the power of one. - **Examples:** 1. Radioactive decay (e.g., $^{14}C \rightarrow ^{14}N + e^-$) 2. Decomposition of N₂O₅: $2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)$ - **Rate Constant Expression:** For a reaction $R \rightarrow P$, the rate law is: $$- \frac{d[R]}{dt} = k[R]$$ Integrating this from $[R]_0$ (initial concentration) at $t=0$ to $[R]_t$ (concentration at time $t$) gives: $$\ln[R]_t - \ln[R]_0 = -kt$$ Or, $$k = \frac{1}{t} \ln \frac{[R]_0}{[R]_t}$$ Where $k$ is the rate constant. ### Sulphuric Acid (Contact Process) - **Principle:** Catalytic oxidation of SO₂ to SO₃ using V₂O₅ as catalyst, followed by absorption of SO₃ in H₂SO₄ to form oleum, which is then diluted. - **Reactions:** 1. **Burner:** $S_8(s) + 8O_2(g) \rightarrow 8SO_2(g)$ 2. **Catalytic Converter (V₂O₅, 400-450°C):** $2SO_2(g) + O_2(g) \rightleftharpoons 2SO_3(g)$ (Exothermic) 3. **Absorption Tower:** $SO_3(g) + H_2SO_4(conc.) \rightarrow H_2S_2O_7$ (Oleum) 4. **Dilution:** $H_2S_2O_7(l) + H_2O(l) \rightarrow 2H_2SO_4(aq)$ - **Conc. H₂SO₄ with Oxalic Acid:** $$(COOH)_2 + H_2SO_4(conc.) \xrightarrow{\Delta} CO + CO_2 + H_2SO_4 \cdot H_2O$$ (Conc. H₂SO₄ acts as a dehydrating agent) ### Sulphuric Acid (Lead Chamber Process) - **Principle:** Oxidation of SO₂ to SO₃ using oxides of nitrogen (NO, NO₂) as catalysts in lead chambers, followed by absorption in water. - **Key Reactions:** 1. **Formation of NO₂:** $S + O_2 \rightarrow SO_2$; $2NO + O_2 \rightarrow 2NO_2$ 2. **Oxidation of SO₂:** $SO_2 + NO_2 \rightarrow SO_3 + NO$ 3. **Hydrolysis:** $SO_3 + H_2O \rightarrow H_2SO_4$ 4. **Regeneration of NO₂:** $2NO + O_2 \rightarrow 2NO_2$ (Note: Produces less concentrated H₂SO₄, largely replaced by Contact Process) ### Nitric Acid (Ostwald's Process) - **Principle:** Catalytic oxidation of ammonia to nitric oxide, followed by oxidation of nitric oxide to nitrogen dioxide, and finally absorption of nitrogen dioxide in water to form nitric acid. - **Reactions:** 1. **Catalytic Oxidation (Pt/Rh gauze, 800°C):** $4NH_3(g) + 5O_2(g) \xrightarrow{Pt/Rh} 4NO(g) + 6H_2O(g)$ 2. **Oxidation of NO:** $2NO(g) + O_2(g) \rightarrow 2NO_2(g)$ 3. **Absorption in Water:** $3NO_2(g) + H_2O(l) \rightarrow 2HNO_3(aq) + NO(g)$ (NO is recycled) - **White Phosphorus and Conc. HNO₃:** $$P_4 + 20HNO_3(conc.) \rightarrow 4H_3PO_4 + 20NO_2 + 4H_2O$$ (Conc. HNO₃ acts as a strong oxidizing agent) ### Batteries - **Definition:** A device that converts chemical energy directly into electrical energy through redox reactions. - **Types:** - **Primary Cells:** Non-rechargeable. Once reactants are consumed, the battery dies. - **Example:** Dry cell (Leclanché cell), Mercury cell. - **Secondary Cells:** Rechargeable. Chemical reactions can be reversed by applying external electrical energy. - **Example:** Lead-acid battery, Lithium-ion battery, Ni-Cd battery. ### Emulsion - **Definition:** A heterogeneous mixture of two immiscible liquids, where one liquid is dispersed in the other in the form of fine droplets. - **Types:** 1. **Oil in Water (O/W):** Oil dispersed as droplets in water. - **Example:** Milk (fat droplets in water), Vanishing cream. 2. **Water in Oil (W/O):** Water dispersed as droplets in oil. - **Example:** Butter (water droplets in oil), Cold cream. ### Elevation of Boiling Point - **Definition:** The increase in the boiling point of a solvent when a non-volatile solute is dissolved in it. $$\Delta T_b = T_b - T_b^0$$ where $T_b$ is the boiling point of solution and $T_b^0$ is the boiling point of pure solvent. - **Mathematical Expression for Molecular Weight:** $$\Delta T_b = K_b \cdot m$$ where $K_b$ is the ebullioscopic constant (molal elevation constant) and $m$ is the molality of the solution. Molality $m = \frac{w_2/M_2}{w_1/1000}$ (where $w_2$ = mass of solute, $M_2$ = molar mass of solute, $w_1$ = mass of solvent in g). Substituting $m$: $$\Delta T_b = K_b \cdot \frac{w_2 \times 1000}{M_2 \times w_1}$$ Rearranging to find $M_2$: $$M_2 = \frac{K_b \times w_2 \times 1000}{\Delta T_b \times w_1}$$ ### Osmotic Pressure - **Definition:** The excess pressure that must be applied to a solution to prevent the passage of solvent molecules into the solution through a semipermeable membrane. - **Colligative Property Proof:** Osmotic pressure ($\Pi$) is given by the van't Hoff equation for dilute solutions: $$\Pi = CRT$$ where $C$ is the molar concentration of the solute, $R$ is the gas constant, and $T$ is the absolute temperature. Since $C = \frac{n_2}{V}$ (moles of solute $n_2$ per volume of solution $V$), $$\Pi = \frac{n_2}{V} RT$$ This equation shows that osmotic pressure depends only on the number of moles of solute ($n_2$) and not on its identity. Therefore, osmotic pressure is a colligative property. ### Roasting & Calcination - **Roasting:** Heating an ore (usually sulfide ore) strongly in the presence of air or oxygen below its melting point. - **Purpose:** Converts sulfide ores to oxides, removes volatile impurities (e.g., As, S). - **Example:** $2ZnS(s) + 3O_2(g) \xrightarrow{\Delta} 2ZnO(s) + 2SO_2(g)$ - **Calcination:** Heating an ore (usually carbonate or hydroxide ore) strongly in the absence or limited supply of air below its melting point. - **Purpose:** Decomposes carbonates to oxides, removes volatile matter (e.g., CO₂, H₂O). - **Example:** $CaCO_3(s) \xrightarrow{\Delta} CaO(s) + CO_2(g)$ ### Mineral & Ore - **Mineral:** A naturally occurring chemical substance, generally inorganic, with a definite chemical composition and a characteristic crystalline structure. - **Example:** Bauxite ($Al_2O_3 \cdot 2H_2O$), Clay ($Al_2O_3 \cdot 2SiO_2 \cdot 2H_2O$). - **Ore:** A mineral from which one or more metals can be extracted profitably and conveniently. All ores are minerals, but not all minerals are ores. - **Example:** Bauxite is an ore of Aluminum, but clay is a mineral of Aluminum but not an ore because extraction is not profitable. ### Absorption & Adsorption - **Absorption:** A bulk phenomenon where a substance (absorbate) is uniformly distributed throughout the bulk of another substance (absorbent). - **Example:** Water vapor absorbed by anhydrous CaCl₂, sponge soaking up water. - **Adsorption:** A surface phenomenon where a substance (adsorbate) accumulates only on the surface of another substance (adsorbent). - **Example:** Ammonia gas adsorbed on charcoal, silica gel adsorbing water vapor. ### Physical Adsorption & Chemical Adsorption | Feature | Physical Adsorption (Physisorption) | Chemical Adsorption (Chemisorption) | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Nature of Forces**| Weak van der Waals forces | Strong chemical bonds (covalent or ionic) | | **Heat of Adsorption**| Low (20-40 kJ/mol) | High (80-240 kJ/mol) | | **Reversibility** | Reversible | Irreversible | | **Layers** | Multimolecular layers | Unimolecular layer | | **Specificity** | Not specific | Highly specific | | **Temperature** | Favored by low temperature | Favored by high temperature (initially, then decreases)| ### Schottky Defect & Frenkel Defect | Feature | Schottky Defect | Frenkel Defect | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Definition** | Pair of cation and anion missing from lattice sites. | Ion leaves its lattice site and occupies an interstitial site. | | **Density** | Decreases density of the crystal. | Does not change density of the crystal. | | **Electrical Neutrality**| Maintained. | Maintained. | | **Ionic Size** | Occurs in compounds with similar cation and anion sizes (e.g., NaCl, KCl). | Occurs in compounds with large difference in ionic sizes (e.g., AgCl, ZnS). | ### Colligative Property - **Definition:** Properties of dilute solutions that depend only on the number of solute particles (moles or molecules) present, irrespective of their nature, and not on the chemical identity of the solute. - **Examples:** 1. Relative lowering of vapor pressure 2. Elevation of boiling point 3. Depression of freezing point 4. Osmotic pressure ### Lyophobic & Lyophilic Colloids | Feature | Lyophilic Colloids (Solvent-loving) | Lyophobic Colloids (Solvent-hating) | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Preparation** | Easily prepared by direct mixing. | Requires special methods (e.g., chemical, dispersion). | | **Stability** | Highly stable (due to strong interaction with dispersion medium). | Less stable, easily coagulated. | | **Reversibility** | Reversible (can be reformed by adding dispersion medium). | Irreversible. | | **Viscosity** | Much higher than dispersion medium. | Nearly same as dispersion medium. | | **Surface Tension** | Lower than dispersion medium. | Nearly same as dispersion medium. | | **Example** | Starch in water, gelatin, gum. | Metal sols (Au sol), metal sulfides. | ### Methanoic Acid & Ethanoic Acid | Feature | Methanoic Acid (Formic Acid) | Ethanoic Acid (Acetic Acid) | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Formula** | HCOOH | CH₃COOH | | **Structure** | Contains both -COOH and -CHO groups (aldehyde & carboxylic). | Contains only -COOH group. | | **Reducing Property**| Strong reducing agent (due to -CHO part), reduces Tollen's and Fehling's reagents. | Does not reduce Tollen's or Fehling's reagents. | | **Oxidation** | Easily oxidized to CO₂ and H₂O. | Less easily oxidized. | | **Preparation** | From CO and NaOH under pressure, followed by H₂SO₄. | From ethanol oxidation, or carbonylation of methanol. | ### Transition Elements (Complexes & Color) - **Why form complex compounds?** 1. **Small size and high nuclear charge:** Allows them to attract and bind with ligands (electron pair donors). 2. **Presence of vacant d-orbitals:** Can accept electron pairs from ligands to form coordinate bonds. 3. **Variable oxidation states:** Allows them to form complexes with various ligands. - **Why form colored compounds?** 1. **Partially filled (n-1)d orbitals:** Most transition metal ions have incompletely filled d-orbitals. 2. **d-d transitions:** When ligands approach the central metal ion, the d-orbitals split into different energy levels. Electrons can absorb specific wavelengths of visible light to jump from a lower energy d-orbital to a higher energy d-orbital (d-d transition). 3. **Complementary color:** The color observed is the complementary color of the light absorbed. ### Power Alcohol & van't Hoff Factor - **Power Alcohol:** Ethanol mixed with gasoline (petrol) in certain proportions (e.g., 5-25%) and used as a fuel for internal combustion engines. Anhydrous ethanol is preferred. - **Advantages:** Renewable, burns cleaner, reduces pollution. - **Disadvantages:** Lower energy content than gasoline, corrosive to some engine parts. - **van't Hoff Factor ($i$):** A measure of the effect of a solute on colligative properties. It is the ratio of the observed colligative property to the theoretical colligative property if the solute were a non-electrolyte. $$i = \frac{\text{Observed Colligative Property}}{\text{Calculated Colligative Property (assuming no dissociation/association)}}$$ - **For non-electrolytes:** $i=1$ - **For electrolytes that dissociate:** $i > 1$ (e.g., NaCl $i \approx 2$) - **For solutes that associate:** $i ### Double Salt & Complex Salt | Feature | Double Salt | Complex Salt (Coordination Compound) | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Definition** | Addition compounds that exist only in solid state and dissociate completely into constituent ions in solution. | Addition compounds that retain their identity in solution; complex ion does not dissociate into simple ions. | | **Stability** | Stable only in solid state. | Stable in both solid and solution states. | | **Identity in Solution**| Loses its identity, gives tests for all constituent ions. | Retains its identity, does not give tests for all constituent ions (e.g., [Fe(CN)₆]³⁻ does not give Fe³⁺ or CN⁻ tests). | | **Bonding** | Ionic bonds between simple ions. | Coordinate bonds between central metal ion and ligands. | | **Example** | Mohr's salt ($FeSO_4 \cdot (NH_4)_2SO_4 \cdot 6H_2O$), Carnallite ($KCl \cdot MgCl_2 \cdot 6H_2O$). | Potassium ferrocyanide ($K_4[Fe(CN)_6]$), Tetramminecopper(II) sulfate ($[Cu(NH_3)_4]SO_4$). | ### Ideal & Non-Ideal Solutions | Feature | Ideal Solutions | Non-Ideal Solutions | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Raoult's Law** | Obeys Raoult's law over entire range of concentrations. | Deviates from Raoult's law. | | **$\Delta H_{mix}$**| $\Delta H_{mix} = 0$ (no heat absorbed or released). | $\Delta H_{mix} \ne 0$ (heat absorbed or released). | | **$\Delta V_{mix}$**| $\Delta V_{mix} = 0$ (no volume change on mixing). | $\Delta V_{mix} \ne 0$ (volume change on mixing). | | **Interactions** | A-A, B-B, and A-B interactions are similar. | A-B interactions are different from A-A and B-B. | | **Deviations** | None | **Positive Deviation:** Vapor pressure higher than expected (e.g., ethanol + acetone). **Negative Deviation:** Vapor pressure lower than expected (e.g., chloroform + acetone). | ### Soap & Cleaning Action - **Soap:** Sodium or potassium salts of long-chain fatty acids (e.g., sodium stearate, $C_{17}H_{35}COONa$). - **How it works in cleaning clothes:** 1. **Structure:** Soap molecules have two parts: a long non-polar hydrocarbon tail (hydrophobic, oil-soluble) and a short polar ionic head (hydrophilic, water-soluble). 2. **Micelle Formation:** When soap is added to water, the hydrophobic tails surround oil/grease particles, while the hydrophilic heads face outwards into the water. This forms a spherical structure called a micelle. 3. **Emulsification:** The micelles trap the oil/grease particles, preventing them from coalescing. The water-soluble outer surface of the micelle allows the entire structure to be suspended in water. 4. **Washing Away:** Agitation (washing) dislodges the micelles containing the dirt, which are then rinsed away with water, cleaning the clothes. ### Principal Ores and Their Compositions | Metal | Ore Name | Composition | | :---------- | :-------------- | :------------------------------------------ | | **Aluminum**| Bauxite | $Al_2O_3 \cdot 2H_2O$ | | | Corundum | $Al_2O_3$ | | **Iron** | Hematite | $Fe_2O_3$ | | | Magnetite | $Fe_3O_4$ | | | Siderite | $FeCO_3$ | | **Copper** | Copper Pyrites | $CuFeS_2$ | | | Cuprite | $Cu_2O$ | | | Malachite | $CuCO_3 \cdot Cu(OH)_2$ | | **Zinc** | Zinc Blende | $ZnS$ | | | Calamine | $ZnCO_3$ | | **Lead** | Galena | $PbS$ | | | Cerussite | $PbCO_3$ | | **Tin** | Cassiterite | $SnO_2$ | | **Gold** | Native Gold | $Au$ (often with Ag) | | **Silver** | Argentite | $Ag_2S$ | | | Horn Silver | $AgCl$ | ### Particles of Unit Cell | Unit Cell Type | Particle at Corners | Particle at Body Center | Particle at Face Center | Total Atoms per Unit Cell | | :--------------- | :------------------ | :---------------------- | :---------------------- | :------------------------ | | **Simple Cubic (SC)**| 1/8 × 8 = 1 | 0 | 0 | 1 | | **Body-Centered Cubic (BCC)**| 1/8 × 8 = 1 | 1 | 0 | 2 | | **Face-Centered Cubic (FCC)**| 1/8 × 8 = 1 | 0 | 1/2 × 6 = 3 | 4 | ### Crystalline & Amorphous Solids | Feature | Crystalline Solids | Amorphous Solids | | :------------------ | :--------------------------------------------------- | :----------------------------------------------------- | | **Arrangement** | Ordered, long-range arrangement of constituent particles. | Disordered, short-range arrangement (random). | | **Melting Point** | Sharp and definite melting point. | Melt over a range of temperature (soften gradually). | | **Cleavage** | Cleave into two pieces with smooth, definite surfaces. | Cleave into two pieces with irregular surfaces. | | **Anisotropy** | Anisotropic (physical properties differ with direction). | Isotropic (physical properties are same in all directions). | | **Nature** | True solids. | Pseudo-solids or supercooled liquids. | | **Heat of Fusion** | Definite heat of fusion. | No definite heat of fusion. | | **Example** | NaCl, Quartz, Diamond. | Glass, Rubber, Plastics. |