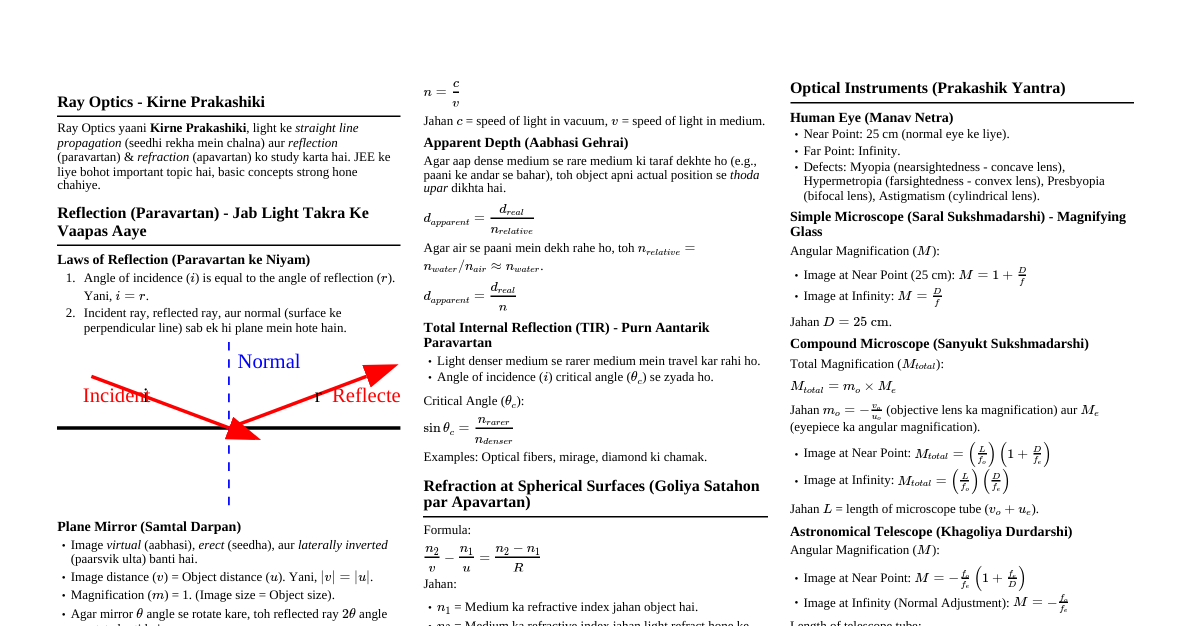
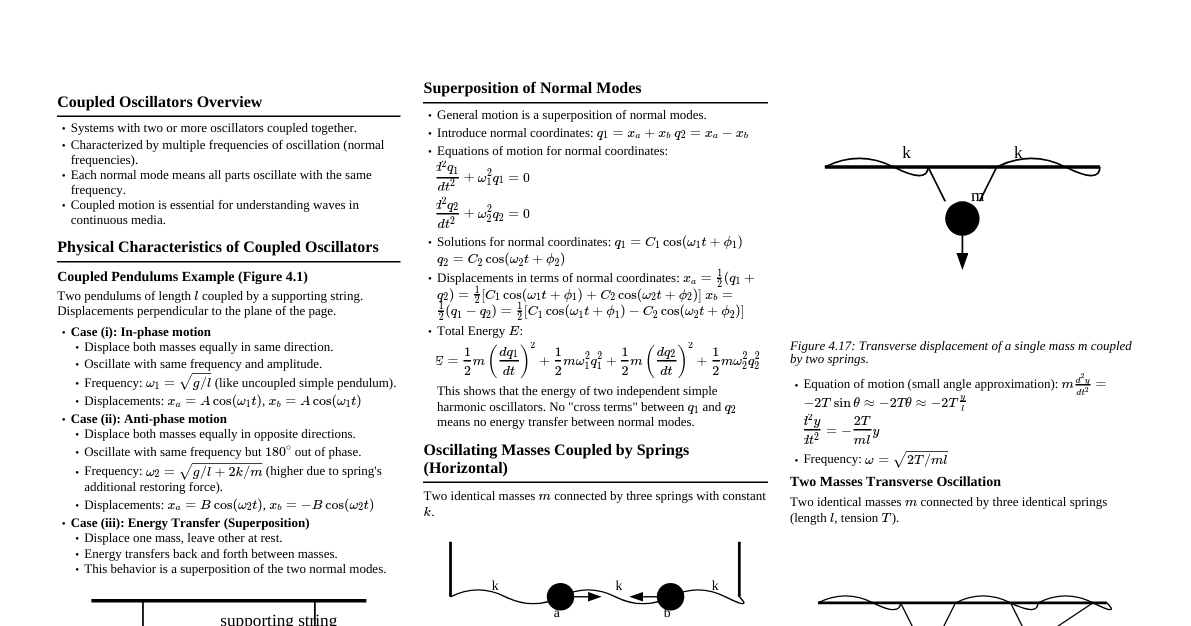
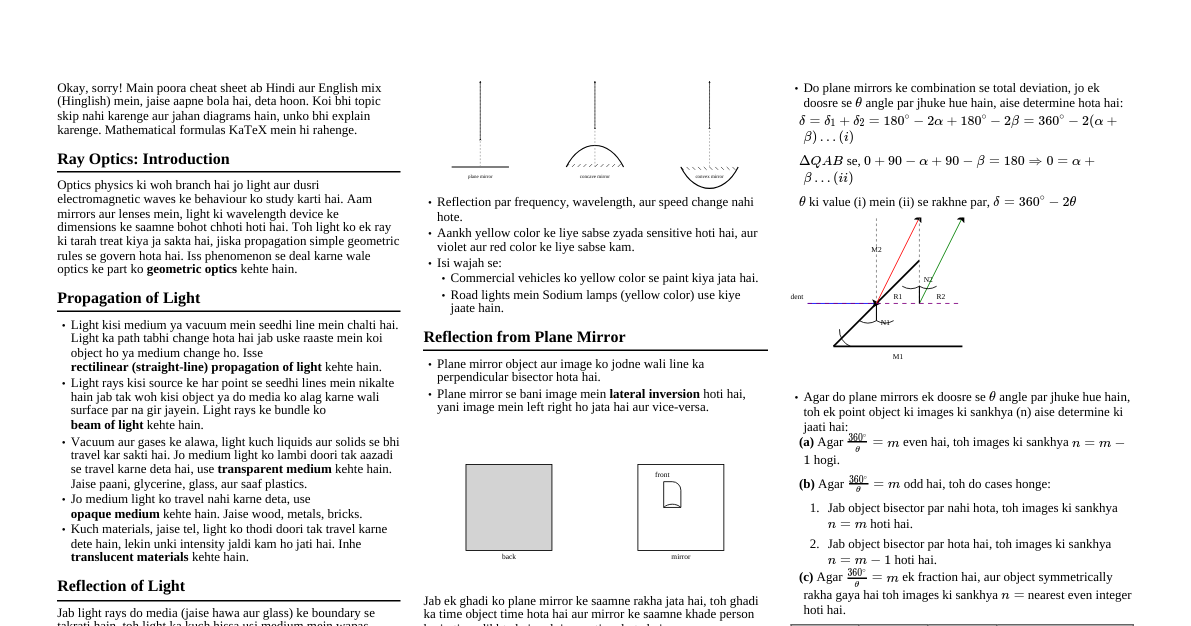
1. Hydrostatics 1.1 Pressure in Fluids Definition: Fluids are substances that can flow. Hydrostatics is the study of fluids at rest. Thrust: The total normal force exerted by a fluid on a surface. Pressure (P): The thrust exerted per unit area. It is a scalar quantity. $$P = \frac{F_{\text{normal}}}{A}$$ SI Unit: Pascal (Pa), where $1 \text{ Pa} = 1 \text{ N/m}^2$. 1.2 Pressure Variation with Depth Pressure $P$ in a fluid of uniform density $\rho$ increases with depth $h$: $$P = P_0 + h\rho g$$ where $P_0$ is the pressure at the surface, $g$ is acceleration due to gravity. The term $h\rho g$ is gauge pressure. 1.3 Hydrostatic Paradox The pressure at a certain depth depends only on the depth and fluid nature, not on container shape or total fluid amount. 2. Pascal's Law and Archimedes' Principle 2.1 Pascal's Law Definition: A pressure change applied to an enclosed, incompressible fluid is transmitted undiminished to every portion of the fluid and to the walls of the containing vessel. Application: Hydraulic Lift: A small force $F_1$ on area $A_1$ creates pressure $P = F_1/A_1$. This pressure is transmitted to a larger piston of area $A_2$, generating a larger force $F_2 = P \times A_2$. $$\frac{F_1}{A_1} = \frac{F_2}{A_2} \implies F_2 = F_1 \left(\frac{A_2}{A_1}\right)$$ Since $A_2 > A_1$, the force is multiplied. 2.2 Archimedes' Principle Definition: When a body is partially or fully immersed in a fluid, it experiences an upward buoyant force equal in magnitude to the weight of the fluid displaced by the body. Buoyant Force ($F_B$): $$F_B = V_{\text{submerged}} \cdot \rho_{\text{fluid}} \cdot g$$ where $V_{\text{submerged}}$ is the volume of the submerged part and $\rho_{\text{fluid}}$ is the fluid density. The buoyant force acts at the center of buoyancy. 3. Floatation and Density 3.1 Laws of Floatation For a body of weight $W$ immersed in a fluid with maximum buoyant force $F_B$: If $W > F_B$, the body will sink. If $W If $W = F_B$, the body will float fully submerged. In floating condition, body weight equals weight of displaced fluid. 3.2 Fraction of Volume Submerged For a floating body of volume $V_{\text{body}}$ and density $\rho_{\text{body}}$ in a fluid of density $\rho_{\text{fluid}}$: $$\frac{V_{\text{submerged}}}{V_{\text{body}}} = \frac{\rho_{\text{body}}}{\rho_{\text{fluid}}}$$ Fraction of volume outside the fluid: $$\frac{V_{\text{out}}}{V_{\text{body}}} = 1 - \frac{\rho_{\text{body}}}{\rho_{\text{fluid}}}$$ 3.3 Density of Mixtures Mixing by Mass: For liquids with masses $m_1, m_2$ and densities $\rho_1, \rho_2$: $$\rho_{\text{mixture}} = \frac{m_1 + m_2}{\frac{m_1}{\rho_1} + \frac{m_2}{\rho_2}}$$ If $m_1 = m_2$, $\rho_{\text{mixture}} = \frac{2\rho_1\rho_2}{\rho_1+\rho_2}$ (harmonic mean). Mixing by Volume: For liquids with volumes $V_1, V_2$ and densities $\rho_1, \rho_2$: $$\rho_{\text{mixture}} = \frac{\rho_1V_1 + \rho_2V_2}{V_1 + V_2}$$ If $V_1 = V_2$, $\rho_{\text{mixture}} = \frac{\rho_1+\rho_2}{2}$ (arithmetic mean). 4. Hydrodynamics 4.1 Fluid Flow and Equation of Continuity Definition: Hydrodynamics is the study of fluids in motion. Streamline Flow: Every particle follows the same path; smooth and orderly. Turbulent Flow: Irregular, disordered flow at high velocities. Ideal Fluid: Incompressible (constant density) and non-viscous (no internal friction). Equation of Continuity: For an ideal fluid in streamline flow, the product of cross-sectional area ($A$) and fluid velocity ($v$) is constant: $$A_1v_1 = A_2v_2 \quad \text{or} \quad Av = \text{constant}$$ This implies fluid speeds up in narrower sections and slows down in wider sections. 4.2 Bernoulli's Theorem Definition: For an ideal fluid in streamline flow, the sum of pressure energy, kinetic energy, and potential energy per unit volume is constant: $$P + \frac{1}{2}\rho v^2 + \rho gh = \text{constant}$$ where $P$ is pressure, $\rho$ is density, $v$ is velocity, $g$ is gravity, and $h$ is height. This is a statement of energy conservation for a flowing fluid. Applications: For horizontal flow ($h=\text{constant}$), $P + \frac{1}{2}\rho v^2 = \text{constant}$. High velocity means low pressure. Explains lift on airplane wings, atomizers, and Magnus effect. 4.3 Torricelli's Theorem (Velocity of Efflux) The speed of liquid flowing out of a small hole at depth $h$ below the free surface is: $$v_{\text{efflux}} = \sqrt{2gh}$$ 5. Viscosity and Stoke's Law 5.1 Viscosity Definition: Viscosity is the fluid property representing internal friction, resistance to flow. Newton's Law of Viscosity: Viscous force $F$ between fluid layers: $$F = -\eta A \frac{dv}{dx}$$ where $\eta$ is the coefficient of viscosity, $A$ is the area, and $dv/dx$ is the velocity gradient. 5.2 Stoke's Law The viscous drag force $F_D$ on a small sphere of radius $r$ moving with velocity $v$ through a fluid of viscosity $\eta$: $$F_D = 6\pi\eta rv$$ 5.3 Terminal Velocity ($v_T$) When an object falls through a viscous fluid, it reaches a constant velocity where gravity balances buoyancy and viscous drag. Key Formula for a sphere: For a sphere of density $\rho$ and radius $r$ falling through a fluid of density $\sigma$ and viscosity $\eta$: $$v_T = \frac{2r^2(\rho - \sigma)g}{9\eta}$$ 6. Surface Tension 6.1 Molecular Forces and Surface Tension Cohesive Force: Attraction between molecules of the same substance. Adhesive Force: Attraction between molecules of different substances. Surface Tension (S): Property of a liquid resisting external force due to molecular cohesion, tending to minimize surface area. 6.2 Surface Tension Definition As force per unit length acting on an imaginary line on the liquid's surface: $$S = \frac{F}{L}$$ As work done (or surface energy) per unit increase in surface area: $$S = \frac{W}{\Delta A}$$ SI Unit: N/m or J/m$^2$. 6.3 Factors Affecting Surface Tension Temperature: Generally decreases as temperature increases. Impurities: Soluble impurities (like salt) increase surface tension, partially soluble impurities (like soap) decrease it. 7. Surface Energy and Excess Pressure 7.1 Work Done and Surface Energy Work done to increase surface area $\Delta A$ is stored as surface potential energy: $$W = \Delta U = S \cdot \Delta A$$ For a liquid drop of radius $R$ (one surface): $\Delta A = 4\pi R^2$. For a soap bubble of radius $R$ (two surfaces): $\Delta A = 2 \times 4\pi R^2 = 8\pi R^2$. 7.2 Excess Pressure Inside a Drop/Bubble Pressure inside a curved liquid surface is greater than outside due to surface tension. Inside a Liquid Drop: $\Delta P = P_{\text{in}} - P_{\text{out}} = \frac{2S}{R}$ Inside a Soap Bubble: $\Delta P = P_{\text{in}} - P_{\text{out}} = \frac{4S}{R}$ (factor of 4 because a bubble has two surfaces). 8. Angle of Contact and Capillarity 8.1 Angle of Contact ($\theta$) Definition: Angle between the tangent to the liquid surface at the point of contact and the solid surface inside the liquid. Meniscus Shape: Concave Meniscus ($\theta Cohesive forces (e.g., water on glass). Convex Meniscus ($\theta > 90^\circ$): Cohesive forces > Adhesive forces (e.g., mercury on glass). 8.2 Capillarity Definition: Phenomenon of liquid rise or fall in a narrow tube (capillary tube) due to surface tension. Capillary Rise (Jurin's Law): Height $h$ to which a liquid of density $\rho$ and surface tension $S$ rises in a capillary tube of radius $r$: $$h = \frac{2S \cos \theta}{r\rho g}$$ If $\theta If $\theta > 90^\circ$ ($\cos \theta$ is negative), liquid is depressed. 9. Fundamental Concepts of Elasticity 9.1 Definitions Elasticity: Property of a body to regain its original shape/size after removal of deforming force. Plasticity: Property of a body not to regain its original shape/size after removal of deforming force. Elastic Limit: Maximum deforming force up to which a body exhibits elasticity. Beyond this, permanent deformation occurs. 9.2 Stress and Strain Stress: Internal restoring force developed per unit area of a deformed body. $$\text{Stress} = \frac{\text{Restoring Force}}{\text{Area}} = \frac{F}{A}$$ SI Unit: N/m$^2$ or Pascal (Pa). Strain: Fractional change in configuration (length, volume, or shape). $$\text{Strain} = \frac{\text{Change in configuration}}{\text{Original configuration}}$$ Strain is a dimensionless quantity. 10. Moduli of Elasticity 10.1 Hooke's Law Within the elastic limit, stress is directly proportional to strain: $$\text{Stress} = E \times \text{Strain}$$ where $E$ is the Modulus of Elasticity. 10.2 Types of Moduli Young's Modulus (Y): Ratio of normal stress to longitudinal strain. $$Y = \frac{\text{Normal Stress}}{\text{Longitudinal Strain}} = \frac{F/A}{\Delta L/L} = \frac{FL}{A\Delta L}$$ Bulk Modulus (K): Ratio of volumetric stress (pressure) to volumetric strain. $$K = \frac{\text{Volumetric Stress}}{\text{Volumetric Strain}} = \frac{-\Delta P}{\Delta V/V} = \frac{-\Delta PV}{\Delta V}$$ Reciprocal of Bulk Modulus is Compressibility ($C = 1/K$). Shear Modulus or Modulus of Rigidity ($\eta$): Ratio of tangential stress to shearing strain. $$\eta = \frac{\text{Tangential Stress}}{\text{Shearing Strain}} = \frac{F/A}{\theta}$$ 10.3 Poisson's Ratio ($\sigma$) Definition: When a wire is stretched longitudinally, it contracts laterally. Poisson's ratio is the ratio of lateral strain to longitudinal strain. $$\sigma = \frac{\text{Lateral Strain}}{\text{Longitudinal Strain}} = \frac{-\Delta R/R}{\Delta L/L}$$ Negative sign indicates radius decreases as length increases. Practical value of $\sigma$ lies between 0 and 0.5. 10.4 Relations Between Elastic Moduli $Y = 2\eta(1 + \sigma)$ $Y = 3K(1 - 2\sigma)$ $\frac{9}{Y} = \frac{3}{\eta} + \frac{1}{K}$ 11. Stress-Strain Relationship 11.1 Definition The stress-strain curve is a graphical representation of a material's mechanical properties. 11.2 Interpretation of the Curve Region OA (Elastic): Stress proportional to strain (Hooke's Law holds). Material returns to original shape. Point B (Elastic Limit): Maximum stress material can withstand without permanent deformation. Region BD (Plastic): Material undergoes permanent deformation. Point C (Ultimate Tensile Strength): Maximum stress material can handle before starting to fail. Point D (Fracture Point): Point where material breaks. 12. Elastic Potential Energy 12.1 Elastic Potential Energy (U) Work done against interatomic forces when a wire is stretched, stored as elastic potential energy. Energy stored in a wire stretched by length $\Delta L$: $$U = \frac{1}{2} \times \text{Stretching Force} \times \text{Elongation} = \frac{1}{2} F\Delta L$$ Elastic potential energy per unit volume (energy density): $$\frac{U}{\text{Volume}} = \frac{1}{2} \times \text{Stress} \times \text{Strain} = \frac{1}{2} Y (\text{Strain})^2$$