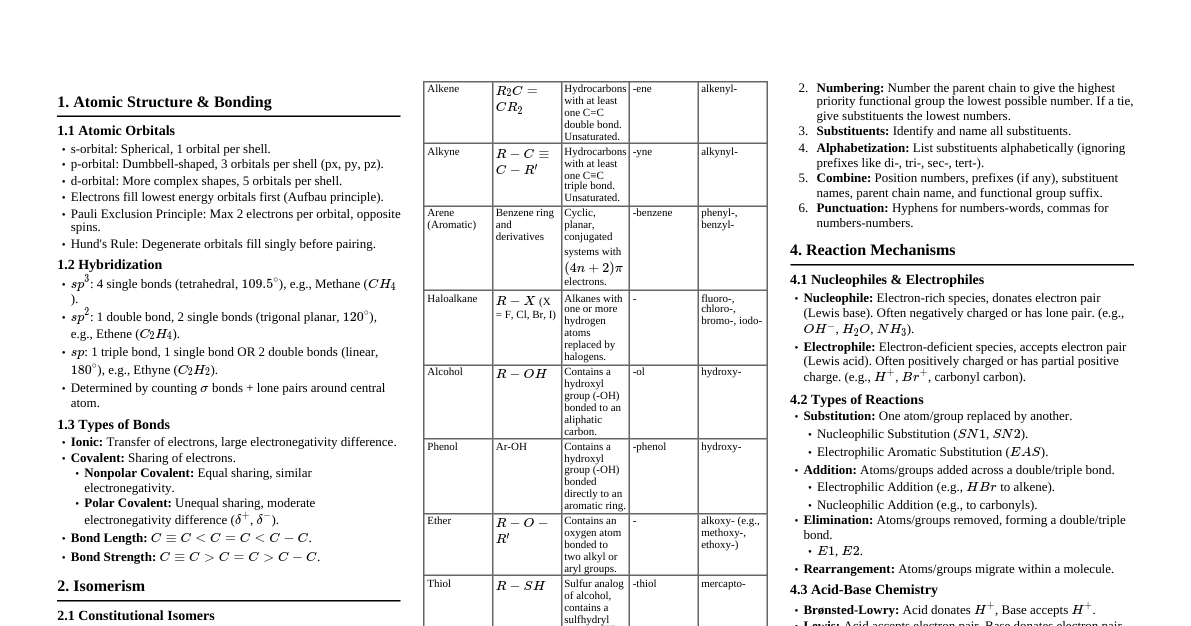
1. Introduction to Hydrocarbons Organic compounds containing only carbon and hydrogen atoms. Classified based on carbon-carbon bonds: Saturated: Alkanes (single bonds) Unsaturated: Alkenes (double bonds), Alkynes (triple bonds) Aromatic: Benzene and its derivatives 2. Alkanes 2.1 General Formula & Structure General formula: $C_nH_{2n+2}$ Saturated acyclic hydrocarbons. Carbon atoms are $sp^3$ hybridized. Tetrahedral geometry around each carbon. Examples: Methane ($CH_4$), Ethane ($C_2H_6$), Propane ($C_3H_8$). 2.2 Isomerism Structural Isomerism: Compounds with the same molecular formula but different structural formulas. Butane ($C_4H_{10}$): n-butane, isobutane (2-methylpropane) Pentane ($C_5H_{12}$): n-pentane, isopentane (2-methylbutane), neopentane (2,2-dimethylpropane) 2.3 Nomenclature (IUPAC) Longest continuous carbon chain. Number chain from end closer to substituent. Alphabetical order for multiple substituents. Prefixes: di-, tri-, tetra- for identical substituents. Example: 2-methylpropane, 2,2,4-trimethylpentane 2.4 Preparation of Alkanes From Unsaturated Hydrocarbons: Hydrogenation (Sabatier-Senderens reaction) $CH_2=CH_2 + H_2 \xrightarrow{Ni/Pt/Pd} CH_3-CH_3$ From Alkyl Halides: Wurtz Reaction: $2RX + 2Na \xrightarrow{dry \ ether} R-R + 2NaX$ (for symmetrical alkanes) Reduction: $R-X + H_2 \xrightarrow{Zn/HCl} R-H + HX$ From Carboxylic Acids: Decarboxylation (Soda lime): $RCOONa + NaOH \xrightarrow{CaO, \Delta} R-H + Na_2CO_3$ Kolbe's Electrolytic Method: $2RCOONa + 2H_2O \xrightarrow{electrolysis} R-R + 2CO_2 + H_2 + 2NaOH$ 2.5 Physical Properties Non-polar, insoluble in water, soluble in organic solvents. Boiling point increases with molecular mass (due to increased van der Waals forces). Branching decreases boiling point (reduced surface area for intermolecular interaction). $C_1-C_4$: gases; $C_5-C_{17}$: liquids; $>C_{18}$: solids. 2.6 Chemical Properties Relatively unreactive (paraffins). Halogenation (Free Radical Substitution): $CH_4 + Cl_2 \xrightarrow{hv} CH_3Cl + HCl$ Mechanism: Initiation ($Cl_2 \xrightarrow{hv} 2Cl \cdot$), Propagation ($Cl \cdot + CH_4 \rightarrow HCl + CH_3 \cdot$; $CH_3 \cdot + Cl_2 \rightarrow CH_3Cl + Cl \cdot$), Termination. Combustion: $CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + Heat$ Controlled Oxidation: $CH_4 + O_2 \xrightarrow{Cu/523K/100atm} CH_3OH$ (Methanol) Isomerisation: n-alkanes to branched alkanes in presence of $AlCl_3/HCl$. Aromatization: Alkanes ($C_6$ or more) to aromatic compounds at high T/P with catalysts ($Cr_2O_3, V_2O_5, Mo_2O_3$). 3. Alkenes 3.1 General Formula & Structure General formula: $C_nH_{2n}$ Unsaturated hydrocarbons with at least one carbon-carbon double bond ($C=C$). Carbon atoms involved in $C=C$ are $sp^2$ hybridized. Planar geometry around each $sp^2$ carbon. Restricted rotation around $C=C$ bond. 3.2 Isomerism Structural Isomerism: Position, chain. Geometrical (cis-trans) Isomerism: Due to restricted rotation around $C=C$ bond. Occurs when each carbon of the double bond is attached to two different groups. 3.3 Nomenclature (IUPAC) Suffix '-ene'. Longest chain containing $C=C$ bond. Number chain to give $C=C$ bond lowest possible number. Example: Ethene, Propene, But-1-ene, But-2-ene. 3.4 Preparation of Alkenes From Alkynes: Partial hydrogenation: $RC \equiv CR' + H_2 \xrightarrow{Pd/BaSO_4} RCH=CHR'$ (Lindlar's catalyst gives cis-alkene) $RC \equiv CR' + Na/liq. NH_3 \rightarrow RCH=CHR'$ (Birch reduction gives trans-alkene) From Alkyl Halides (Dehydrohalogenation): $RCH_2CH_2X + alc. KOH \rightarrow RCH=CH_2 + KX + H_2O$ Follows Saytzeff's Rule: More substituted alkene is major product. From Alcohols (Dehydration): $RCH_2CH_2OH \xrightarrow{conc. H_2SO_4, \Delta} RCH=CH_2 + H_2O$ Follows Saytzeff's Rule. From Vicinal Dihalides (Dehalogenation): $RCHBr-CH_2Br + Zn \rightarrow RCH=CH_2 + ZnBr_2$ 3.5 Physical Properties Similar to alkanes. Non-polar, insoluble in water. $C_2-C_4$: gases; $C_5-C_{17}$: liquids; $>C_{18}$: solids. 3.6 Chemical Properties Undergo electrophilic addition reactions due to $\pi$-electron cloud. Addition of Hydrogen (Hydrogenation): $CH_2=CH_2 + H_2 \xrightarrow{Ni/Pt/Pd} CH_3-CH_3$ Addition of Halogens: $CH_2=CH_2 + Br_2 \rightarrow CH_2Br-CH_2Br$ (Decolorizes bromine water, test for unsaturation) Addition of Hydrogen Halides (HX): $CH_2=CH_2 + HBr \rightarrow CH_3CH_2Br$ For unsymmetrical alkenes, follows Markovnikov's Rule: Negative part of addendum adds to carbon with fewer hydrogen atoms. $CH_3CH=CH_2 + HBr \rightarrow CH_3CH(Br)CH_3$ (major) Anti-Markovnikov's Rule (Peroxide Effect/Kharasch effect) for HBr in presence of peroxides. Addition of Water: $CH_2=CH_2 + H_2O \xrightarrow{H^+} CH_3CH_2OH$ (Hydration) Ozonolysis: Cleaves $C=C$ bond to form aldehydes and ketones. $R_2C=CR_2 \xrightarrow{O_3} \text{Ozonide intermediate} \xrightarrow{Zn/H_2O} R_2C=O + O=CR_2$ Polymerization: Formation of long chain polymers. Example: Polyethene from ethene. 4. Alkynes 4.1 General Formula & Structure General formula: $C_nH_{2n-2}$ Unsaturated hydrocarbons with at least one carbon-carbon triple bond ($C \equiv C$). Carbon atoms involved in $C \equiv C$ are $sp$ hybridized. Linear geometry around each $sp$ carbon. Example: Ethyne ($C_2H_2$, Acetylene) 4.2 Acidity of Terminal Alkynes Terminal alkynes (e.g., $CH \equiv CH$, $CH_3C \equiv CH$) have acidic hydrogen due to high s-character of $sp$ hybridized carbon. They react with strong bases and form metal acetylides. $CH \equiv CH + Na \rightarrow CH \equiv C^-Na^+ + 1/2 H_2$ $CH \equiv CH + Ag(NH_3)_2^+ \rightarrow CH \equiv C^-Ag^+ \downarrow$ (White ppt with Tollen's reagent) 4.3 Preparation of Alkynes From Calcium Carbide: $CaC_2 + 2H_2O \rightarrow Ca(OH)_2 + CH \equiv CH$ From Vicinal Dihalides: Double dehydrohalogenation $CH_2Br-CH_2Br + 2KOH(alc.) \rightarrow CH \equiv CH + 2KBr + 2H_2O$ (requires strong base like $NaNH_2$) 4.4 Chemical Properties Undergo electrophilic addition reactions, similar to alkenes, but in two steps. Addition of Hydrogen: $CH \equiv CH + H_2 \xrightarrow{Pd/BaSO_4} CH_2=CH_2 \xrightarrow{H_2} CH_3-CH_3$ (Complete hydrogenation) Addition of Halogens: $CH \equiv CH + Br_2 \rightarrow CHBr=CHBr \xrightarrow{Br_2} CHBr_2-CHBr_2$ Addition of Hydrogen Halides: Follows Markovnikov's rule. $CH \equiv CH + HBr \rightarrow CH_2=CHBr \xrightarrow{HBr} CH_3-CHBr_2$ Addition of Water (Hydration): $CH \equiv CH + H_2O \xrightarrow{HgSO_4/H_2SO_4} CH_2=CHOH \text{ (enol)} \rightarrow CH_3CHO \text{ (acetaldehyde)}$ Polymerization: Linear polymerization: $nCH \equiv CH \xrightarrow{Cu_2Cl_2} (-CH=CH-CH=CH-)_n$ (polyacetylene) Cyclic polymerization: $3CH \equiv CH \xrightarrow{red \ hot \ Fe \ tube} C_6H_6$ (Benzene) 5. Aromatic Hydrocarbons (Benzene) 5.1 Introduction Contain one or more benzene rings. Benzene ($C_6H_6$) is the simplest aromatic hydrocarbon. Possess special stability due to delocalization of $\pi$-electrons (aromaticity). Hückel's Rule: $(4n+2)\pi$ electrons. 5.2 Structure of Benzene Planar hexagonal ring. All C-C bond lengths are equal (139 pm), intermediate between single (154 pm) and double (134 pm). All C-H bond lengths are equal (109 pm). Each carbon is $sp^2$ hybridized. Each carbon has one unhybridized p-orbital, which overlap to form delocalized $\pi$-electron cloud above and below the ring. 5.3 Preparation of Benzene Cyclic Polymerization of Ethyne: $3CH \equiv CH \xrightarrow{red \ hot \ Fe \ tube} C_6H_6$ Decarboxylation of Benzoic Acid: $C_6H_5COONa + NaOH \xrightarrow{CaO, \Delta} C_6H_6 + Na_2CO_3$ Reduction of Phenol: $C_6H_5OH + Zn \rightarrow C_6H_6 + ZnO$ 5.4 Chemical Properties Undergo electrophilic substitution reactions, not addition reactions (to maintain aromaticity). Nitration: $C_6H_6 + HNO_3 \xrightarrow{conc. H_2SO_4} C_6H_5NO_2 + H_2O$ (Nitrobenzene) Halogenation: $C_6H_6 + Cl_2 \xrightarrow{FeCl_3} C_6H_5Cl + HCl$ (Chlorobenzene) Sulphonation: $C_6H_6 + H_2SO_4 \xrightarrow{\Delta} C_6H_5SO_3H + H_2O$ (Benzenesulphonic acid) Friedel-Crafts Alkylation: $C_6H_6 + R-Cl \xrightarrow{anhyd. AlCl_3} C_6H_5-R + HCl$ (e.g., Toluene from $CH_3Cl$) Friedel-Crafts Acylation: $C_6H_6 + RCOCl \xrightarrow{anhyd. AlCl_3} C_6H_5COR + HCl$ (e.g., Acetophenone from $CH_3COCl$) Addition Reactions (under extreme conditions): Hydrogenation: $C_6H_6 + 3H_2 \xrightarrow{Ni, \Delta} C_6H_{12}$ (Cyclohexane) Chlorination: $C_6H_6 + 3Cl_2 \xrightarrow{hv} C_6H_6Cl_6$ (Benzene Hexachloride, BHC) 5.5 Directing Groups in Substituted Benzene Ortho-para directing & Activating: Groups that increase electron density on ortho and para positions and activate the ring towards electrophilic substitution. Examples: $-OH, -NH_2, -OR, -NHR, -CH_3, -R, -X$ (halogens are deactivating but o,p-directing) Meta-directing & Deactivating: Groups that decrease electron density on ortho and para positions (making meta position relatively richer) and deactivate the ring. Examples: $-NO_2, -CN, -CHO, -COR, -COOH, -COOR, -SO_3H$