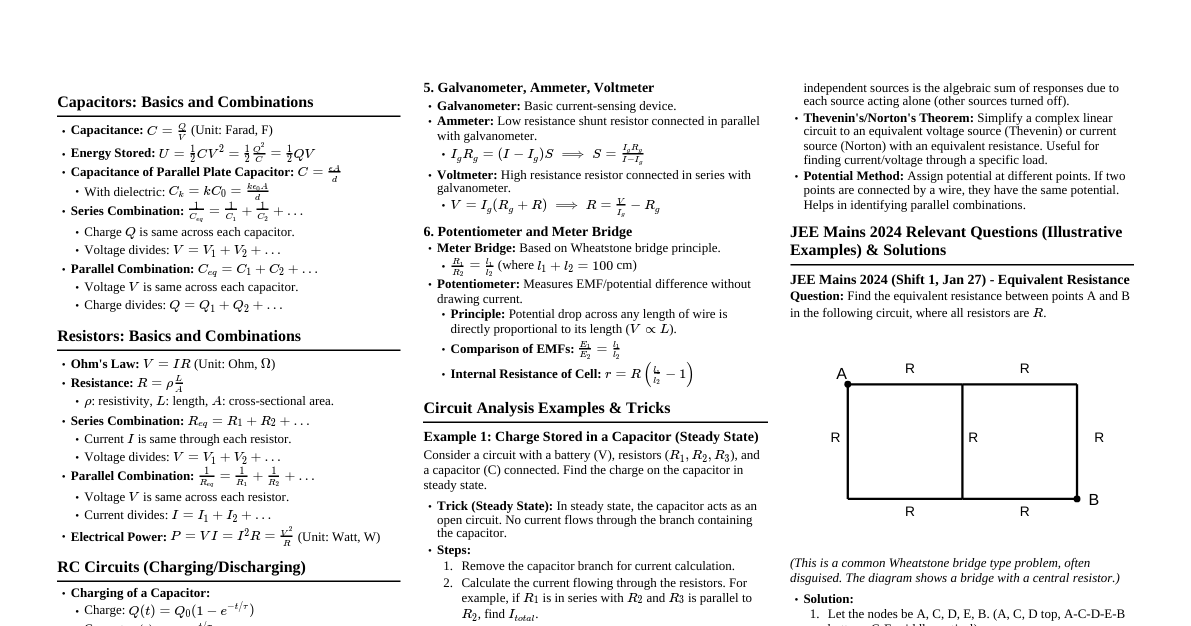
### Introduction: Your 2-Month JEE Battle Plan This cheatsheet is designed to be your ultimate companion for cracking JEE Mains and Advanced in just two months. It covers essential topics from Physics, Chemistry, and Mathematics, provides quick revision formulas, short tricks, and a structured timetable. Success in JEE requires consistent effort, smart study, and effective time management. Let's conquer it! ### 2-Month Study Timetable (Daily Structure) This timetable is a general guideline. Adjust it based on your strengths and weaknesses. #### Week 1-4: Concept Building & Problem Solving - **Morning (6:00 AM - 9:00 AM):** Physics - New Topic / Revision + PYQs - **Break (9:00 AM - 9:30 AM):** Refreshment - **Late Morning (9:30 AM - 12:30 PM):** Chemistry - New Topic / Revision + PYQs - **Lunch & Rest (12:30 PM - 2:00 PM):** Recharge - **Afternoon (2:00 PM - 5:00 PM):** Mathematics - New Topic / Revision + PYQs - **Evening (5:00 PM - 6:00 PM):** Short Break / Light Activity - **Late Evening (6:00 PM - 9:00 PM):** Mixed Subject Practice, Doubts, Short Tricks, Mind Map Creation - **Night (9:00 PM - 10:00 PM):** Revision of the day's topics, Formula Recall - **Sleep (10:00 PM onwards):** Minimum 7-8 hours #### Week 5-6: Intensive Revision & Mock Tests - **Daily:** 1 Full Mock Test (JEE Mains/Advanced alternate days) - **Post-Mock Analysis:** 3-4 hours dedicated to analyzing mistakes, revising concepts, and solving incorrect questions. - **Remaining Time:** Focused revision of weak areas, solving high-weightage PYQs, short tricks practice. #### Week 7-8: Final Polish & Strategy - **Daily:** 1 Full Mock Test (Focus on JEE Advanced pattern in Week 7, JEE Mains in Week 8) - **Post-Mock Analysis:** Intensive analysis, error logging, strategy refinement. - **Revision:** High-yield topics, formula sheets, mind maps. - **Mental Preparation:** Visualization, stress management. - **Last few days:** Only light revision, no new topics, focus on confidence. **Key:** - **PYQs:** Previous Year Questions - **Mind Maps:** Visual summaries of concepts. ### Physics Essentials (11th & 12th) #### 1. Mechanics - **Kinematics:** $\vec{v} = \vec{u} + \vec{a}t$, $\vec{s} = \vec{u}t + \frac{1}{2}\vec{a}t^2$, $v^2 = u^2 + 2as$. Projectile motion. - **Newton's Laws:** $F=ma$, Impulse $J = \Delta p$. Conservation of momentum. - **Work, Energy, Power:** $W = \vec{F} \cdot \vec{d}$, $KE = \frac{1}{2}mv^2$, $PE = mgh$. Conservation of energy. - **Rotational Motion:** $\tau = I\alpha$, $L = I\omega$. Conservation of angular momentum. - **Gravitation:** $F = \frac{GMm}{r^2}$, $PE = -\frac{GMm}{r}$. Kepler's laws. - **Short Trick:** For inclined plane problems, resolve forces along and perpendicular to the incline. #### 2. Thermodynamics & KTG - **Heat & Temperature:** $Q = mc\Delta T$, Latent Heat $Q=mL$. - **Thermodynamic Processes:** Isothermal, Adiabatic, Isobaric, Isochoric. Work done $W = P\Delta V$. - **First Law:** $\Delta U = Q - W$. - **Second Law:** Entropy. Carnot Engine efficiency $\eta = 1 - \frac{T_C}{T_H}$. - **KTG:** $P = \frac{1}{3} \frac{mN}{V} v_{rms}^2$. Average KE per molecule = $\frac{3}{2}kT$. #### 3. Waves & Oscillations - **SHM:** $x = A\sin(\omega t + \phi)$, $v = A\omega\cos(\omega t + \phi)$. Pendulum $T = 2\pi\sqrt{\frac{L}{g}}$. - **Waves:** $v = f\lambda$. Standing waves. Doppler effect $f' = f \frac{v \pm v_o}{v \mp v_s}$. #### 4. Electromagnetism - **Electrostatics:** Coulomb's Law $F = \frac{1}{4\pi\epsilon_0}\frac{q_1q_2}{r^2}$. Electric field $\vec{E}$, potential $V$. Gauss's Law. Capacitance $C = \frac{Q}{V}$. - **Current Electricity:** Ohm's Law $V=IR$. Resistivity $\rho$. Kirchhoff's Laws. Wheatstone bridge. - **Magnetism:** Biot-Savart Law. Ampere's Law. Lorentz Force $\vec{F} = q(\vec{E} + \vec{v} \times \vec{B})$. - **EMI & AC:** Faraday's Law, Lenz's Law. Inductance $L$. LC Oscillations. AC circuits (RLC). - **Short Trick:** For complex circuits, use symmetry or node analysis. #### 5. Optics - **Ray Optics:** Reflection, Refraction (Snell's Law), Lenses, Mirrors. Lens Maker's Formula $\frac{1}{f} = (\mu-1)(\frac{1}{R_1} - \frac{1}{R_2})$. - **Wave Optics:** Interference (Young's Double Slit $y_n = \frac{n\lambda D}{d}$), Diffraction, Polarization. #### 6. Modern Physics - **Dual Nature:** Photoelectric effect $KE_{max} = h\nu - \phi_0$. De Broglie wavelength $\lambda = \frac{h}{p}$. - **Atomic Structure:** Bohr's Model. Hydrogen spectrum. - **Nuclear Physics:** Radioactivity, Binding Energy. Mass defect. Half-life. - **Semiconductors:** Diodes, Transistors (Basic understanding). - **Short Trick:** For photoelectric effect, remember energy conservation. *(Image: Example of a Physics Mind Map for Electromagnetism showing key concepts and formulas.)* ### Chemistry Essentials (11th & 12th) #### 1. Physical Chemistry ##### a. Basic Concepts & Stoichiometry - Mole concept, empirical and molecular formula, limiting reagent. - **Short Trick:** Use mole ratios directly for stoichiometric calculations. ##### b. Atomic Structure - Bohr's model, quantum numbers, Aufbau, Hund's, Pauli's Exclusion. - **Formulas:** $E_n = -13.6 \frac{Z^2}{n^2}$ eV. $\Delta E = h\nu$. ##### c. States of Matter - Ideal gas equation $PV=nRT$. Gas Laws. Van der Waals equation. - Liquid state, solid state (unit cells, packing efficiency). ##### d. Thermodynamics & Energetics - Hess's Law, spontaneity $\Delta G = \Delta H - T\Delta S$. - **Short Trick:** For $\Delta H$ calculations, remember products - reactants. ##### e. Chemical Equilibrium - $K_c, K_p$. Le Chatelier's Principle. - **Ionic Equilibrium:** pH, Buffer solutions, Solubility Product $K_{sp}$. ##### f. Solutions - Raoult's Law, colligative properties (relative lowering of vapor pressure, elevation in boiling point, depression in freezing point, osmotic pressure). ##### g. Electrochemistry - Nernst equation, Gibbs free energy and cell potential $\Delta G = -nFE_{cell}$. - Faraday's laws of electrolysis. ##### h. Chemical Kinetics - Rate laws, order of reaction, half-life. Arrhenius equation. - **Short Trick:** For first-order reactions, $t_{1/2} = \frac{0.693}{k}$. ##### i. Surface Chemistry - Adsorption, colloids, emulsions. #### 2. Inorganic Chemistry ##### a. Periodic Table & Periodicity - Trends in atomic/ionic radii, ionization enthalpy, electron gain enthalpy, electronegativity. ##### b. Chemical Bonding & Molecular Structure - VSEPR theory, hybridization, molecular orbital theory (MOT). - **Short Trick:** For hybridization, count sigma bonds + lone pairs. ##### c. s-Block Elements - Group 1 & 2 elements, properties, compounds. ##### d. p-Block Elements - Group 13 to 18 elements, properties, important compounds (e.g., Boron compounds, Nitrogen compounds, Halogens, Noble gases). ##### e. d- & f-Block Elements - Transition elements, lanthanides, actinides. Coordination compounds (Werner's theory, VBT, CFT, isomerism). ##### f. Metallurgy - Principles and processes of metal extraction. #### 3. Organic Chemistry ##### a. GOC (General Organic Chemistry) - IUPAC nomenclature, isomerism, electronic effects (inductive, resonance, hyperconjugation). - Reaction intermediates (carbocations, carbanions, free radicals). ##### b. Hydrocarbons - Alkanes, alkenes, alkynes (preparation, reactions). Aromatic compounds (Benzene, electrophilic substitution). ##### c. Halogen Derivatives - Alkyl and aryl halides (SN1, SN2, E1, E2 reactions). - **Short Trick:** SN1/E1 prefer 3° carbocations, SN2/E2 prefer less hindered. ##### d. Oxygen Containing Compounds - Alcohols, Phenols, Ethers (preparation, reactions). - Aldehydes, Ketones (nucleophilic addition, oxidation, reduction). - Carboxylic Acids and their derivatives. ##### e. Nitrogen Containing Compounds - Amines (basicity, preparation, reactions), Diazonium salts. ##### f. Biomolecules & Polymers & Chemistry in Everyday Life - Carbohydrates, Proteins, Nucleic Acids, Vitamins. - Classification, preparation, and uses of polymers. - Drugs, detergents, food additives. ##### g. Practical Organic Chemistry - Qualitative and quantitative analysis. *(Image: Example of an Organic Chemistry reaction flowchart connecting different functional groups.)* ### Mathematics Essentials (11th & 12th) #### 1. Algebra ##### a. Complex Numbers - Argand plane, polar form, Euler's form $e^{i\theta} = \cos\theta + i\sin\theta$. De Moivre's Theorem. - **Short Trick:** For roots of unity, visualize on the complex plane. ##### b. Quadratic Equations - Roots, relations between roots and coefficients. Nature of roots. - **Short Trick:** For common roots, use the condition $c_1a_2-c_2a_1)^2 = (a_1b_2-a_2b_1)(b_1c_2-b_2c_1)$. ##### c. Sequences & Series - AP, GP, HP. Sum to $n$ terms. AM-GM inequality. - **Short Trick:** For sum of an AP, $S_n = \frac{n}{2}(a+l)$. ##### d. Permutations & Combinations - Factorial, permutations $P(n,r)$, combinations $C(n,r)$. - **Short Trick:** Remember $P(n,r) = \frac{n!}{(n-r)!}$ and $C(n,r) = \frac{n!}{r!(n-r)!}$. ##### e. Binomial Theorem - General term, middle term, properties of binomial coefficients. - **Short Trick:** $(x+y)^n = \sum_{k=0}^n C(n,k) x^{n-k} y^k$. ##### f. Matrices & Determinants - Types of matrices, operations. Determinants, adjoint, inverse. Cramer's Rule. - **Short Trick:** For $2 \times 2$ inverse: $\frac{1}{ad-bc} \begin{pmatrix} d & -b \\ -c & a \end{pmatrix}$. ##### g. Probability - Classical, conditional probability, Bayes' Theorem. Bernoulli trials, Binomial distribution. #### 2. Calculus ##### a. Functions - Domain, range, types of functions (odd/even, periodic, one-one, onto). Inverse functions. - **Short Trick:** To check injectivity, if $f(x_1)=f(x_2) \implies x_1=x_2$. ##### b. Limits, Continuity & Differentiability - L'Hopital's Rule. Differentiability from first principles. - **Short Trick:** For indeterminate forms, use L'Hopital's. ##### c. Differentiation - Chain rule, product rule, quotient rule. Implicit differentiation. Logarithmic differentiation. - **Formulas:** $\frac{d}{dx}(\sin x) = \cos x$, $\frac{d}{dx}(\tan^{-1} x) = \frac{1}{1+x^2}$. ##### d. Applications of Derivatives - Tangents and Normals, Maxima and Minima, Rate of Change, Rolle's Theorem, Mean Value Theorem. - **Short Trick:** For maxima/minima, $f'(x)=0$ and check $f''(x)$ sign. ##### e. Indefinite Integration - Basic formulas, substitution, integration by parts. - **Short Trick:** $\int e^x (f(x) + f'(x)) dx = e^x f(x) + C$. ##### f. Definite Integration - Properties of definite integrals. Area under curve. - **Short Trick:** $\int_a^b f(x)dx = \int_a^b f(a+b-x)dx$. ##### g. Differential Equations - Order and degree. Variables separable, homogeneous, linear differential equations. #### 3. Coordinate Geometry ##### a. Straight Lines - Slope, various forms of equation of line. Distance between parallel lines. - **Short Trick:** Equation of line passing through $(x_1, y_1)$ with slope $m$: $y-y_1 = m(x-x_1)$. ##### b. Circles - Equation of circle, tangent, normal. - **Short Trick:** Center $(-g, -f)$, radius $\sqrt{g^2+f^2-c}$ for $x^2+y^2+2gx+2fy+c=0$. ##### c. Parabola, Ellipse, Hyperbola - Standard equations, focus, directrix, eccentricity. Tangents and Normals. #### 4. Vector Algebra & 3D Geometry ##### a. Vectors - Dot product, cross product. Scalar triple product, vector triple product. - **Short Trick:** $\vec{a} \cdot \vec{b} = |\vec{a}||\vec{b}|\cos\theta$. $|\vec{a} \times \vec{b}| = |\vec{a}||\vec{b}|\sin\theta$. ##### b. 3D Geometry - Direction cosines, direction ratios. Lines and planes in 3D. Shortest distance between skew lines. #### 5. Statistics & Reasoning - Mean, Median, Mode, Variance, Standard Deviation. - Mathematical Reasoning (Logic gates, truth tables - JEE Mains specific). *(Image: Example of a concise Mathematics formula sheet for Calculus.)* ### PYQs, Mock Tests & Paper Analysis #### 1. Previous Year Questions (PYQs) - **Importance:** PYQs are the most reliable source for understanding exam patterns, difficulty levels, and important topics. - **Strategy:** - Solve PYQs topic-wise immediately after completing a topic. - Solve full-length PYQ papers under timed conditions for the last 4 weeks. - Analyze every question: why you got it right, why you got it wrong, or why you couldn't attempt it. #### 2. Mock Tests - **Frequency:** - **Initial Month:** 1-2 mock tests per week (focus on identifying weak areas). - **Last Month:** 3-4 mock tests per week, increasing to daily in the final two weeks. - **Simulate Exam Conditions:** Take tests at the same time slots as actual JEE exam. Use OMR sheets (for Mains) and proper rough work. - **Types:** Take both JEE Mains and JEE Advanced pattern mock tests. #### 3. Paper Analysis - **The Most Crucial Step:** A mock test is useless without thorough analysis. - **Process:** 1. **Identify Mistakes:** Categorize them (conceptual error, silly mistake, calculation error, time management error, lack of knowledge). 2. **Review Correct Answers:** Ensure you understood the correct approach and if there was a faster method. 3. **Analyze Unattempted Questions:** Why couldn't you attempt them? Lack of knowledge? Too lengthy? 4. **Time Management:** Identify sections where you spent too much or too little time. 5. **Topic-wise Performance:** Note down weak topics that need more revision. 6. **Maintain an Error Log:** Keep a dedicated notebook for your mistakes and their correct solutions/concepts. Revise this regularly. #### 4. Short Tricks & Speed Calculation - Practice mental math. - Learn and apply specific short tricks for each subject (e.g., approximation, elimination of options, specific formulas). - **Example (Physics):** For projectile range on an inclined plane, use specific formulas instead of deriving every time. - **Example (Maths):** For definite integrals with limits 0 to a, use properties like $\int_0^a f(x)dx = \int_0^a f(a-x)dx$. - **Example (Chemistry):** For finding hybridization, count sigma bonds and lone pairs. ### Mind Maps and Visual Aids - **Mind Maps:** Create visual summaries for complex topics. Branch out from a central concept to sub-topics, formulas, and key points. Use different colors and symbols. - **Flashcards:** For formulas, definitions, and chemical reactions. - **Flowcharts:** Especially useful for organic chemistry reaction mechanisms or problem-solving algorithms. - **Diagrams:** Practice drawing diagrams for physics concepts (circuits, ray diagrams) and chemistry (molecular structures, reaction setups). - **Formula Sheets:** Compile a concise list of all essential formulas for quick revision. - **Visual Memory:** Our brain remembers images better than plain text. Utilize this to your advantage. *(Image: A comprehensive formula sheet for all subjects, highlighting key equations.)* ### Final Tips and Strategies 1. **Consistency is Key:** Stick to your timetable as much as possible. 2. **Prioritize Weak Areas:** Dedicate more time to subjects/topics where you struggle. 3. **Health First:** Ensure adequate sleep, nutrition, and short breaks. Avoid burnout. 4. **Stay Positive:** Believe in yourself. A positive mindset can significantly impact performance. 5. **Avoid New Topics in Last Week:** Focus purely on revision and mock tests in the final days. 6. **Revision Strategy:** Active recall (testing yourself), spaced repetition (revisiting topics at increasing intervals). 7. **Exam Day Strategy:** - Read instructions carefully. - Manage your time per section. - Attempt easier questions first to build confidence. - Don't get stuck on one question. - Stay calm and focused. You have two months. Make every day count! Good luck!